Nuclear-mitochondrial DNA segments resemble paternally inherited mitochondrial DNA in humans
- PMID: 32269217
- PMCID: PMC7142097
- DOI: 10.1038/s41467-020-15336-3
Nuclear-mitochondrial DNA segments resemble paternally inherited mitochondrial DNA in humans
Erratum in
-
Author Correction: Nuclear-mitochondrial DNA segments resemble paternally inherited mitochondrial DNA in humans.Nat Commun. 2020 Jul 22;11(1):3741. doi: 10.1038/s41467-020-17572-z. Nat Commun. 2020. PMID: 32699324 Free PMC article.
Abstract
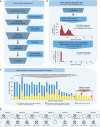
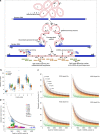
Several strands of evidence question the dogma that human mitochondrial DNA (mtDNA) is inherited exclusively down the maternal line, most recently in three families where several individuals harbored a 'heteroplasmic haplotype' consistent with biparental transmission. Here we report a similar genetic signature in 7 of 11,035 trios, with allelic fractions of 5-25%, implying biparental inheritance of mtDNA in 0.06% of offspring. However, analysing the nuclear whole genome sequence, we observe likely large rare or unique nuclear-mitochondrial DNA segments (mega-NUMTs) transmitted from the father in all 7 families. Independently detecting mega-NUMTs in 0.13% of fathers, we see autosomal transmission of the haplotype. Finally, we show the haplotype allele fraction can be explained by complex concatenated mtDNA-derived sequences rearranged within the nuclear genome. We conclude that rare cryptic mega-NUMTs can resemble paternally mtDNA heteroplasmy, but find no evidence of paternal transmission of mtDNA in humans.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

