Developing a new class of engineered live bacterial therapeutics to treat human diseases
- PMID: 32269218
- PMCID: PMC7142098
- DOI: 10.1038/s41467-020-15508-1
Developing a new class of engineered live bacterial therapeutics to treat human diseases
Abstract
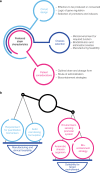
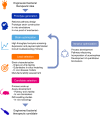
A complex interplay of metabolic and immunological mechanisms underlies many diseases that represent a substantial unmet medical need. There is an increasing appreciation of the role microbes play in human health and disease, and evidence is accumulating that a new class of live biotherapeutics comprised of engineered microbes could address specific mechanisms of disease. Using the tools of synthetic biology, nonpathogenic bacteria can be designed to sense and respond to environmental signals in order to consume harmful compounds and deliver therapeutic effectors. In this perspective, we describe considerations for the design and development of engineered live biotherapeutics to achieve regulatory and patient acceptance.
Conflict of interest statement
The authors declare the following competing interests. M.R.C., V.M.I., N.L., and C.B.K. are all employees of Synlogic, Inc.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

