Polyherbal Medicine Divya Sarva-Kalp-Kwath Ameliorates Persistent Carbon Tetrachloride Induced Biochemical and Pathological Liver Impairments in Wistar Rats and in HepG2 Cells
- PMID: 32269524
- PMCID: PMC7109321
- DOI: 10.3389/fphar.2020.00288
Polyherbal Medicine Divya Sarva-Kalp-Kwath Ameliorates Persistent Carbon Tetrachloride Induced Biochemical and Pathological Liver Impairments in Wistar Rats and in HepG2 Cells
Abstract
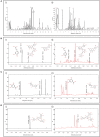
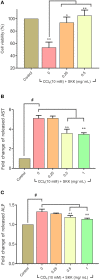
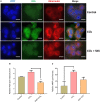
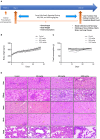
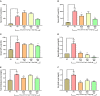
Divya Sarva-Kalp-Kwath (SKK) is a poly-herbal ayurvedic medicine formulated using plant extracts of Boerhavia diffusa L. (Nyctaginaceae), Phyllanthus niruri L. (Euphorbiaceae), and Solanum nigrum L. (Solanaceae), described to improve liver function and general health. In the present study, we have explored the hepatoprotective effects of SKK in ameliorating carbon tetrachloride (CCl4) induced liver toxicity using in-vitro and in-vivo test systems. Chemical analysis of SKK using Liquid Chromatography-Mass Spectroscopy (LC-MS-QToF) and High-Performance Liquid Chromatography (HPLC) revealed the presence of different bioactive plant metabolites, known to impart hepatoprotective effects. In human hepatocarcinoma (HepG2) cells, co-treatment of SKK with CCl4 effectively reduced the hepatotoxicity induced by the latter. These effects were confirmed by studying parameters such as loss of cell viability; release of hepatic injury enzymatic biomarkers- aspartate aminotransferase (AST), and alkaline phosphatase (ALP); and changes in reactive oxygen species and in mitochondrial membrane potentials. In-vivo safety analysis in Wistar rats showed no loss in animal body weight, or change in feeding habits after repeated oral dosing of SKK up to 1,000 mg/kg/day for 28 days. Also, no injury-related histopathological changes were observed in the animal's blood, liver, kidney, heart, brain, and lung. Pharmacologically, SKK played a significant role in modulating CCl4 induced hepatic injuries in the Wistar rats at a higher dose. In the 9 weeks' study, SKK (200 mg/kg) reduced the CCl4 stimulated increase in the release of enzymes (ALT, AST, and ALP), bilirubin, total cholesterol, and uric acid levels in the Wistar rats. It also reduced the CCl4 stimulated inflammatory lesions such as liver fibrosis, lymphocytic infiltration, and hyper-plasticity. In conclusion, SKK showed pharmacological effects in improving the CCl4 stimulated liver injuries in HepG2 cells and in Wistar rats. Furthermore, no adverse effects were observed up to 10× higher human equivalent dose of SKK during 28-days repeated dose exposure in Wistar rats. Based on the literature search on the identified plant metabolites, SKK was found to act in multiple ways to ameliorate CCl4 induced hepatotoxicity. Therefore, polyherbal SKK medicine has shown remarkable potentials as a possible alternative therapeutics for reducing liver toxicity induced by drugs, and other toxins.
Keywords: Divya Sarva-Kalp-Kwath; HepG2 cells; carbon tetrachloride; hepatoprotective effects; hepatotoxicity; safety.
Copyright © 2020 Balkrishna, Sakat, Ranjan, Joshi, Shukla, Joshi, Verma, Gupta, Bhattacharya and Varshney.
Figures


References
-
- Albouchi F., Attia M., Hanana M., Hamrouni L. (2018). Ethnobotanical notes and phytopharmacologiques on Solanum nigrum Linn. (Family: Solanaceae). Am. J. Phytomed. Clin. Ther. 6:5 10.21767/2321-2748.100341 - DOI
-
- Atanu F., Ebiloma G., Ajayi E. (2011). A review of the pharmacological aspects of Solanum nigrum Linn. Biotechnol. Mol. Biol. Rev. 6, 1–7.
LinkOut - more resources
Full Text Sources

