Astrocytogenesis: where, when, and how
- PMID: 32269761
- PMCID: PMC7122459
- DOI: 10.12688/f1000research.22405.1
Astrocytogenesis: where, when, and how
Abstract
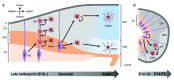
Astrocytes are the most abundant cell type in the central nervous system and have diverse functions in blood-brain barrier maintenance, neural circuitry formation and function, and metabolic regulation. To better understand the diverse roles of astrocytes, we will summarize what is known about astrocyte development and the challenges limiting our understanding of this process. We will also discuss new approaches and technologies advancing the field.
Keywords: Astrocyte; Glia; Gliogenesis; Neurodevelopment.
Copyright: © 2020 Akdemir ES et al.
Conflict of interest statement
No competing interests were disclosed.No competing interests were disclosed.No competing interests were disclosed.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

