Causality assessment of adverse events following immunization: the problem of multifactorial pathology
- PMID: 32269767
- PMCID: PMC7111503
- DOI: 10.12688/f1000research.22600.2
Causality assessment of adverse events following immunization: the problem of multifactorial pathology
Abstract
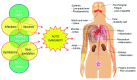
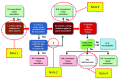
The analysis of Adverse Events Following Immunization (AEFI) is important in a balanced epidemiological evaluation of vaccines and in the issues related to vaccine injury compensation programs. The majority of adverse reactions to vaccines occur as excessive or biased inflammatory and immune responses. These unwanted phenomena, occasionally severe, are associated with many different endogenous and exogenous factors, which often interact in complex ways. The confirmation or denial of the causal link between an AEFI and vaccination is determined pursuant to WHO guidelines, which propose a four-step analysis and algorithmic diagramming. The evaluation process from the onset considers all possible "other causes" that might explain the AEFI and thus exclude the role of the vaccine. Subsequently, even if there was biological plausibility and temporal compatibility for a causal association between the vaccine and the AEFI, the guidelines ask to look for any possible evidence that the vaccine could not have caused that event. Such an algorithmic method presents several concerns that are discussed here, in the light of the multifactorial nature of the inflammatory and immune pathologies induced by vaccines, including emerging knowledge of genetic susceptibility to adverse effects. It is proposed that the causality assessment could exclude a consistent association of the adverse event with the vaccine only when the presumed "other cause" is independent of an interaction with the vaccine. Furthermore, the scientific literature should be viewed not as an exclusion criterion but as a comprehensive analysis of all the evidence for or against the role of the vaccine in causing an adverse reaction. Given these inadequacies in the evaluation of multifactorial diseases, the WHO guidelines need to be reevaluated and revised. These issues are discussed in relation to the laws that, in some countries, regulate the mandatory vaccinations and the compensation for those who have suffered serious adverse effects.
Keywords: Adverse events following immunization; Autoimmunity; Genetic susceptibility; Inflammation; Injury compensation; Mandatory vaccinations; Multifactorial diseases; Vaccination.
Copyright: © 2020 Bellavite P.
Conflict of interest statement
Competing interests: The author has no competing interests. In the past three years he has been involved in some consultancy, conferences and publications in which he has supported the freedom of vaccination. He has always carried out these activities for free and in none of these activities has he received any payment, direct or indirect. In particular, the author wrote a "pro-veritate" memorandum in defence of a doctor in a disciplinary procedure activated by Treviso (I) medical Order to contest positions contrary to indiscriminate vaccinations; advised the Veneto Region on the occasion of the appeal to the Italian Constitutional Court against Law 119/2017; wrote a document on invitation to the Senate Hygiene and Health Commission advocating the cause of vaccination freedom; and recently studied the case of a girl affected by post-vaccination encephalopathy in the request for compensation ex-law 210/1992. The proceeds of his book "Vaccines yes, Obligations no” (Libreria Cortina Editions, Verona, 2017) is entirely donated to a charity association. He does not profit financially in any way from the scientific work on the subject of this article.
Figures

References
-
- WHO: Causality assessment of an adverse event following immunization (AEFI): user manual for the revised WHO classification (Second edition). 2nd Edition, Geneva, World Health Organization.2018. Reference Source
-
- Freckelton I: Vaccination Litigation: The Need for Rethinking Compensation for Victims of Vaccination Injury. J Law Med. 2018;25(2):293–314. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

