Human Derived Immortalized Dermal Papilla Cells With a Constant Expression of Testosterone Receptor
- PMID: 32269992
- PMCID: PMC7109449
- DOI: 10.3389/fcell.2020.00157
Human Derived Immortalized Dermal Papilla Cells With a Constant Expression of Testosterone Receptor
Abstract
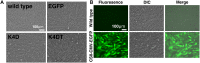
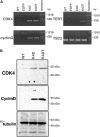
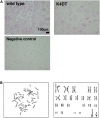
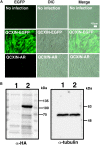
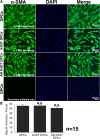
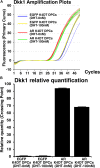
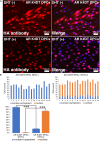
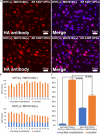
Androgenetic alopecia (AGA) is the most common type of hair loss, and is mainly caused by the biological effects of testosterone on dermal papilla cells (DPCs). In vitro culturing of DPCs might be a useful tool for the screening of target molecule of AGA. However, primary DPCs cannot continuously proliferate owing to cellular senescence and cell culture stress. In this study, we introduced mutant cyclin-dependent kinase 4 (CDK4), Cyclin D1, and telomerase reverse transcriptase (TERT) into DPCs. We confirmed protein expression of CDK4 and Cyclin D1, and enzymatic activity of TERT. Furthermore, we found the established cell line was free from cellular senescence. We also introduced the androgen receptor gene using a recombinant retrovirus, to compensate the transcriptional suppressed endogenous androgen receptor in the process of cell proliferation. Furthermore, we detected the efficient nuclear translocation of androgen receptor into the nucleus after the treatment of dihydrotestosterone, indicating the functionality of our introduced receptor. Our established cell line is a useful tool to identify the downstream signaling pathway, which activated by the testosterone.
Keywords: androgen receptor; dermal papilla cells; dihydrotestosterone; immortalization; nuclear localization.
Copyright © 2020 Fukuda, Takahashi, Takase, Orimoto, Eitsuka, Nakagawa and Kiyono.
Figures



References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

