The Influence of Neuron-Extrinsic Factors and Aging on Injury Progression and Axonal Repair in the Central Nervous System
- PMID: 32269994
- PMCID: PMC7109259
- DOI: 10.3389/fcell.2020.00190
The Influence of Neuron-Extrinsic Factors and Aging on Injury Progression and Axonal Repair in the Central Nervous System
Abstract
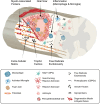
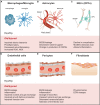
In the aging western population, the average age of incidence for spinal cord injury (SCI) has increased, as has the length of survival of SCI patients. This places great importance on understanding SCI in middle-aged and aging patients. Axon regeneration after injury is an area of study that has received substantial attention and made important experimental progress, however, our understanding of how aging affects this process, and any therapeutic effort to modulate repair, is incomplete. The growth and regeneration of axons is mediated by both neuron intrinsic and extrinsic factors. In this review we explore some of the key extrinsic influences on axon regeneration in the literature, focusing on inflammation and astrogliosis, other cellular responses, components of the extracellular matrix, and myelin proteins. We will describe how each element supports the contention that axonal growth after injury in the central nervous system shows an age-dependent decline, and how this may affect outcomes after a SCI.
Keywords: aging; astrocytes; extra-cellular matrix; inflammation; microglia; neuron-extrinsic factors; signaling; spinal cord injury.
Copyright © 2020 Sutherland and Geoffroy.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources

