Cassava shrunken-2 homolog MeAPL3 determines storage root starch and dry matter content and modulates storage root postharvest physiological deterioration
- PMID: 32270429
- PMCID: PMC9163024
- DOI: 10.1007/s11103-020-00995-z
Cassava shrunken-2 homolog MeAPL3 determines storage root starch and dry matter content and modulates storage root postharvest physiological deterioration
Abstract
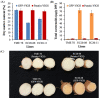
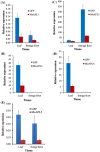
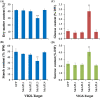
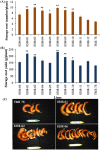
Among the five cassava isoforms (MeAPL1-MeAPL5), MeAPL3 is responsible for determining storage root starch content. Degree of storage root postharvest physiological deterioration (PPD) is directly correlated with starch content. AGPase is heterotetramer composed of two small and two large subunits each coded by small gene families in higher plants. Studies in cassava (Manihot esculenta) identified and characterized five isoforms of Manihot esculenta ADP-glucose pyrophosphorylase large subunit (MeAPL1-MeAPL5) and employed virus induced gene silencing (VIGS) to show that MeAPL3 is the key isoform responsible for starch and dry matter accumulation in cassava storage roots. Silencing of MeAPL3 in cassava through stable transgenic lines resulted in plants displaying significant reduction in storage root starch and dry matter content (DMC) and induced a distinct phenotype associated with increased petiole/stem angle, resulting in a droopy leaf phenotype. Plants with reduced starch and DMC also displayed significantly reduced or no postharvest physiological deterioration (PPD) compared to controls and lines with high DMC and starch content. This provides strong evidence for direct relationships between starch/dry matter content and its role in PPD and canopy architecture traits in cassava.
Keywords: ADP-glucose pyrophophorylase; Cassava; Dry matter; MeAPL3; Postharvest physiological deterioration; Starch.
© 2020. The Author(s).
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures




References
-
- AACC . Approved methods of the American association of cereal chemists. St. Paul: American Association of Cereal Chemists; 2000.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

