Long-Lived Charge-Transfer State Induced by Spin-Orbit Charge Transfer Intersystem Crossing (SOCT-ISC) in a Compact Spiro Electron Donor/Acceptor Dyad
- PMID: 32270586
- PMCID: PMC7496792
- DOI: 10.1002/anie.202003560
Long-Lived Charge-Transfer State Induced by Spin-Orbit Charge Transfer Intersystem Crossing (SOCT-ISC) in a Compact Spiro Electron Donor/Acceptor Dyad
Abstract
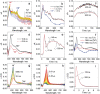
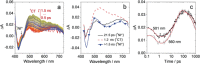
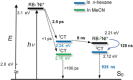
We prepared conceptually novel, fully rigid, spiro compact electron donor (Rhodamine B, lactam form, RB)/acceptor (naphthalimide; NI) orthogonal dyad to attain the long-lived triplet charge-transfer (3 CT) state, based on the electron spin control using spin-orbit charge transfer intersystem crossing (SOCT-ISC). Transient absorption (TA) spectra indicate the first charge separation (CS) takes place within 2.5 ps, subsequent SOCT-ISC takes 8 ns to produce the 3 NI* state. Then the slow secondary CS (125 ns) gives the long-lived 3 CT state (0.94 μs in deaerated n-hexane) with high energy level (ca. 2.12 eV). The cascade photophysical processes of the dyad upon photoexcitation are summarized as 1 NI*→1 CT→3 NI*→3 CT. With time-resolved electron paramagnetic resonance (TREPR) spectra, an EEEAAA electron-spin polarization pattern was observed for the naphthalimide-localized triplet state. Our spiro compact dyad structure and the electron spin-control approach is different to previous methods for which invoking transition-metal coordination or chromophores with intrinsic ISC ability is mandatory.
Keywords: charge transfer; electron spin control; intersystem crossing; time-resolved EPR; triplet state.
© 2020 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Koshiyama T., Shirai M., Hikage T., Tabe, Tanaka H. K., Kitagawa S., Ueno T., Angew. Chem. Int. Ed. 2011, 50, 4849–4852; - PubMed
- Angew. Chem. 2011, 123, 4951–4954.
-
- None
-
- Lim G. N., Obondi C. O., D'Souza F., Angew. Chem. Int. Ed. 2016, 55, 11517–11521; - PubMed
- Angew. Chem. 2016, 128, 11689–11693;
-
- Shao S., Thomas M. B., Park K. H., Mahaffey Z., Kim D., D'Souza F., Chem. Commun. 2018, 54, 54–57. - PubMed
-
- Ziessel R., Allen B. D., Rewinska D. B., Harriman A., Chem. Eur. J. 2009, 15, 7382–7393. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

