Impact of CYP2C9-Interacting Drugs on Warfarin Pharmacogenomics
- PMID: 32270628
- PMCID: PMC7485961
- DOI: 10.1111/cts.12781
Impact of CYP2C9-Interacting Drugs on Warfarin Pharmacogenomics
Abstract
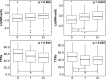
Precise dosing of warfarin is important to achieve therapeutic benefit without adverse effects. Pharmacogenomics explains some interindividual variability in warfarin response, but less attention has been paid to drug-drug interactions in the context of genetic factors. We investigated retrospectively the combined effects of cytochrome P450 (CYP)2C9 and vitamin K epoxide reductase complex (VKORC)1 genotypes and concurrent exposure to CYP2C9-interacting drugs on long-term measures of warfarin anticoagulation. Study participants predicted to be sensitive responders to warfarin based on CYP2C9 and VKORC1 genotypes, had significantly greater international normalized ratio (INR) variability over time. Participants who were concurrently taking CYP2C9-interacting drugs were found to have greater INR variability and lesser time in therapeutic range. The associations of INR variability with genotype were driven by the subgroup not exposed to interacting drugs, whereas the effect of interacting drug exposure was driven by the subgroup categorized as normal responders. Our findings emphasize the importance of considering drug interactions in pharmacogenomic studies.
© 2020 The Authors. Clinical and Translational Science published by Wiley Periodicals, Inc. on behalf of the American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
Dr. George serves on Scientific Advisory Boards for Amgen, Inc. and Otsuka Pharmaceuticals, and received grant funding from Praxis Precision Medicines, Inc. All other authors declared no competing interests for this work.
Figures



References
-
- Pirmohamed, M. et al A randomized trial of genotype‐guided dosing of warfarin. N. Engl. J. Med. 369, 2294–2303 (2013). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

