Evolution of Anion Relay Chemistry: Construction of Architecturally Complex Natural Products
- PMID: 32270672
- PMCID: PMC7301606
- DOI: 10.1021/acs.accounts.0c00076
Evolution of Anion Relay Chemistry: Construction of Architecturally Complex Natural Products
Abstract
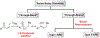
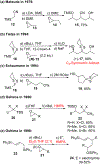
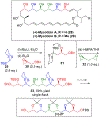
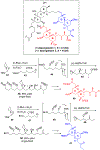
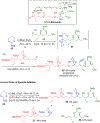
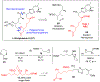
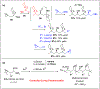
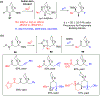
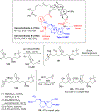
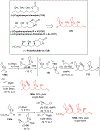
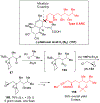
Multicomponent union tactics in which three or more fragments are rapidly connected are highly prized in the construction of architecturally complex natural products. Anion Relay Chemistry (ARC), a multicomponent union tactic, has just such potential to elaborate structurally diverse scaffolds in a single operation with excellent stereochemical control. Conceptually, the ARC tactic can be divided into two main classes: "Through-Bond," by the relay of negative charge through the bonding system of a molecule; and "Through-Space," by the migration of negative charge across space by a transfer agent. "Through-Space" Anion Relay Chemistry, the focus of this Account, can be further subdivided into two types: Type I ARC, originated from the Tietze-Schaumann-Smith coupling reaction, which for the first time permits controllable Brook rearrangements to construct unsymmetrical adducts, and as such has been successfully employed in the total syntheses of diverse natural products, including the mycoticins, bryostatin 1, spongistatins, rimocidin, indolizidine alkaloids, and enigmazole A; and Type II ARC, central to which is the design of novel bifunctional linchpins that enable rapid assembly of linear and cyclic fragments with diverse architectural features, ranging from polyols, spiroketals, and polyenes to polypropionate scaffolds. Recently, the Type II ARC tactic has been exploited as the key construction tactic in the total syntheses of the spirastrellolides, the cryptocarya acetates, secu'amamine A, mandelalide A, and nahuoic acid Ci (Bii). This Account will present the evolution of both the Type I and Type II Anion Relay tactics, in conjunction with some prominent applications.
Conflict of interest statement
The authors declare no competing financial interest.
Figures















References
-
- Bertz SH Complexity of Synthetic Routes: Linear, Convergent and Reflexive Syntheses. New. J. Chem 2003, 27, 870–879.
-
- Graaff C; Ruijter E; Orru RVA Recent Developments in Asymmetric Multicomponent Reactions. Chem. Soc. Rev 2012, 41, 3969–4009 and references therein. - PubMed
-
- Brook AG Isomerism of Some α-Hydroxysilanes to Silyl Ethers. J. Am. Chem. Soc 1958, 80, 1886–1889.
-
- Gilman H; Wu TC Reactions of Triphenylsilylpotassium with Benzophenone and with 4,4’-Dimethylbenzophenone. J. Am. Chem. Soc 1953, 75, 2935–2936.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

