Inorganic polyphosphate is produced and hydrolyzed in F0F1-ATP synthase of mammalian mitochondria
- PMID: 32270854
- PMCID: PMC7200627
- DOI: 10.1042/BCJ20200042
Inorganic polyphosphate is produced and hydrolyzed in F0F1-ATP synthase of mammalian mitochondria
Abstract
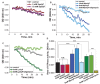
Inorganic polyphosphate (polyP) is a polymer present in all living organisms. Although polyP is found to be involved in a variety of functions in cells of higher organisms, the enzyme responsible for polyP production and consumption has not yet been identified. Here, we studied the effect of polyP on mitochondrial respiration, oxidative phosphorylation and activity of F0F1-ATPsynthase. We have found that polyP activates mitochondrial respiration which does not coupled with ATP production (V2) but inhibits ADP-dependent respiration (V3). Moreover, PolyP can stimulate F0F1-ATPase activity in the presence of ATP and, importantly, can be hydrolyzed in this enzyme instead of ATP. Furthermore, PolyP can be produced in mitochondria in the presence of substrates for respiration and phosphate by the F0F1-ATPsynthase. Thus, polyP is an energy molecule in mammalian cells which can be produced and hydrolyzed in the mitochondrial F0F1-ATPsynthase.
Keywords: F0-F1-ATPase; bioenergetics; inorganic polyphosphates; mitochondria.
© 2020 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

