Recent Progress on Cellulose-Based Ionic Compounds for Biomaterials
- PMID: 32270900
- PMCID: PMC11469321
- DOI: 10.1002/adma.202000717
Recent Progress on Cellulose-Based Ionic Compounds for Biomaterials
Abstract
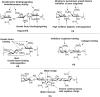
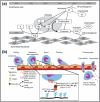
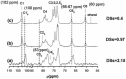
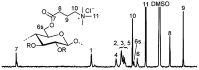
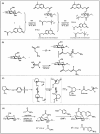
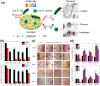
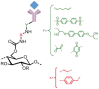
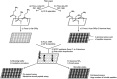
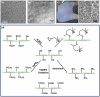
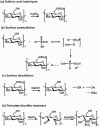
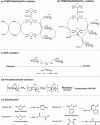
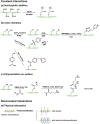
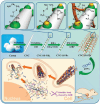
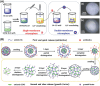
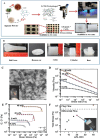
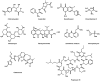
Glycans play important roles in all major kingdoms of organisms, such as archea, bacteria, fungi, plants, and animals. Cellulose, the most abundant polysaccharide on the Earth, plays a predominant role for mechanical stability in plants, and finds a plethora of applications by humans. Beyond traditional use, biomedical application of cellulose becomes feasible with advances of soluble cellulose derivatives with diverse functional moieties along the backbone and modified nanocellulose with versatile functional groups on the surface due to the native features of cellulose as both cellulose chains and supramolecular ordered domains as extractable nanocellulose. With the focus on ionic cellulose-based compounds involving both these groups primarily for biomedical applications, a brief introduction about glycoscience and especially native biologically active glycosaminoglycans with specific biomedical application areas on humans is given, which inspires further development of bioactive compounds from glycans. Then, both polymeric cellulose derivatives and nanocellulose-based compounds synthesized as versatile biomaterials for a large variety of biomedical applications, such as for wound dressings, controlled release, encapsulation of cells and enzymes, and tissue engineering, are separately described, regarding the diverse routes of synthesis and the established and suggested applications for these highly interesting materials.
Keywords: biomaterials; cellulose; ionic; nanocellulose.
© 2020 The Authors. Published by WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
-
- Moon R. J., Martini A., Nairn J., Simonsen J., Youngblood J., Chem. Soc. Rev. 2011, 40, 3941. - PubMed
-
- Fagette P., ASAIO J. 1999, 45, 238. - PubMed
-
- Lavanya D., Kulkarni P., Dixit M., Raavi P. K., Krishna L. N. V., Int. J. Drug Formulation Res. 2011, 2, 2.
-
- Mahiout A., Meinhold H., Kessel M., Schulze H., Baurmeister U., Artif. Organs 1987, 11, 149. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

