Virulence of the emerging pathogen, Burkholderia pseudomallei, depends upon the O-linked oligosaccharyltransferase, PglL
- PMID: 32271107
- PMCID: PMC7611010
- DOI: 10.2217/fmb-2019-0165
Virulence of the emerging pathogen, Burkholderia pseudomallei, depends upon the O-linked oligosaccharyltransferase, PglL
Abstract
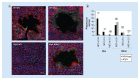
Aim: We sought to characterize the contribution of the O-OTase, PglL, to virulence in two Burkholderia spp. by comparing isogenic mutants in Burkholderia pseudomallei with the related species, Burkholderia thailandensis. Materials & methods: We utilized an array of in vitro assays in addition to Galleria mellonella and murine in vivo models to assess virulence of the mutant and wild-type strains in each Burkholderia species. Results: We found that pglL contributes to biofilm and twitching motility in both species. PglL uniquely affected morphology; cell invasion; intracellular motility; plaque formation and intergenus competition in B. pseudomallei. This mutant was attenuated in the murine model, and extended survival in a vaccine-challenge experiment. Conclusion: Our data support a broad role for pglL in bacterial fitness and virulence, particularly in B. pseudomallei.
Keywords: Burkholderia; PglL; glycosylation; oligosaccharyltransferase; secretion system; virulence.
Conflict of interest statement
Financial & competing interests disclosure
We thank the Wellcome Trust, UK for funding this research (grant ref 102979/Z/13/Z). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Figures

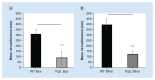

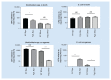
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
