IMPDH inhibitors for antitumor therapy in tuberous sclerosis complex
- PMID: 32271165
- PMCID: PMC7205253
- DOI: 10.1172/jci.insight.135071
IMPDH inhibitors for antitumor therapy in tuberous sclerosis complex
Abstract
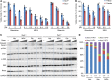
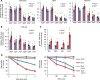
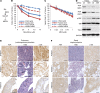
Recent studies in distinct preclinical tumor models have established the nucleotide synthesis enzyme inosine-5'-monophosphate dehydrogenase (IMPDH) as a viable target for antitumor therapy. IMPDH inhibitors have been used clinically for decades as safe and effective immunosuppressants. However, the potential to repurpose these pharmacological agents for antitumor therapy requires further investigation, including direct comparisons of available compounds. Therefore, we tested structurally distinct IMPDH inhibitors in multiple cell and mouse tumor models of the genetic tumor syndrome tuberous sclerosis complex (TSC). TSC-associated tumors are driven by uncontrolled activation of the growth-promoting protein kinase complex mechanistic target of rapamycin (mTOR) complex 1 (mTORC1), which is also aberrantly activated in the majority of sporadic cancers. Despite eliciting similar immunosuppressive effects, the IMPDH inhibitor mizoribine, used clinically throughout Asia, demonstrated far superior antitumor activity compared with the FDA-approved IMPDH inhibitor mycophenolate mofetil (or CellCept, a prodrug of mycophenolic acid). When compared directly to the mTOR inhibitor rapamycin, mizoribine treatment provided a more durable antitumor response associated with tumor cell death. These results provide preclinical support for repurposing mizoribine, over other IMPDH inhibitors, as an alternative to mTOR inhibitors for the treatment of TSC-associated tumors and possibly other tumors featuring uncontrolled mTORC1 activity.
Keywords: Cancer; Genetic diseases; Metabolism; Therapeutics; Tumor suppressors.
Conflict of interest statement
Figures





References
-
- Valvezan AJ, Manning BD. Molecular logic of mTORC1 signalling as a metabolic rheostat. Nat Metab. 2019;1(1):321–333. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

