Electro-Osmotic Vortices Promote the Capture of Folded Proteins by PlyAB Nanopores
- PMID: 32271587
- PMCID: PMC7227020
- DOI: 10.1021/acs.nanolett.0c00877
Electro-Osmotic Vortices Promote the Capture of Folded Proteins by PlyAB Nanopores
Abstract
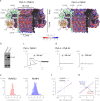
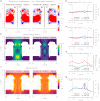
Biological nanopores are emerging as powerful tools for single-molecule analysis and sequencing. Here, we engineered the two-component pleurotolysin (PlyAB) toxin to assemble into 7.2 × 10.5 nm cylindrical nanopores with a low level of electrical noise in lipid bilayers, and we addressed the nanofluidic properties of the nanopore by continuum simulations. Surprisingly, proteins such as human albumin (66.5 kDa) and human transferrin (76-81 kDa) did not enter the nanopore. We found that the precise engineering of the inner surface charge of the PlyAB induced electro-osmotic vortices that allowed the electrophoretic capture of the proteins. Once inside the nanopore, two human plasma proteins could be distinguished by the characteristics of their current blockades. This fundamental understanding of the nanofluidic properties of nanopores provides a practical method to promote the capture and analysis of folded proteins by nanopores.
Keywords: biological nanopore; electro-osmosis; nanofluidics; plasma proteins; single-molecule sensing.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

