Randomized Double-Blind Phase II Study of Maintenance Pembrolizumab Versus Placebo After First-Line Chemotherapy in Patients With Metastatic Urothelial Cancer
- PMID: 32271672
- PMCID: PMC7255983
- DOI: 10.1200/JCO.19.03091
Randomized Double-Blind Phase II Study of Maintenance Pembrolizumab Versus Placebo After First-Line Chemotherapy in Patients With Metastatic Urothelial Cancer
Abstract
Purpose: Platinum-based chemotherapy for first-line treatment of metastatic urothelial cancer is typically administered for a fixed duration followed by observation until progression. "Switch maintenance" therapy with PD-1 blockade at the time of chemotherapy cessation may be attractive for mechanistic and pragmatic reasons.
Patients and methods: Patients with metastatic urothelial cancer achieving at least stable disease on first-line platinum-based chemotherapy were enrolled. Patients were randomly assigned double-blind 1:1 to switch maintenance pembrolizumab 200 mg intravenously once every 3 weeks versus placebo for up to 24 months. Patients with disease progression on placebo could cross over to pembrolizumab. The primary objective was to determine the progression-free survival. Secondary objectives included determining overall survival as well as treatment outcomes according to PD-L1 combined positive score (CPS).
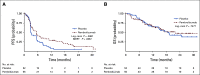
Results: Between December 2015 and November 2018, 108 patients were randomly assigned to pembrolizumab (n = 55) or placebo (n = 53). The objective response rate was 23% with pembrolizumab and 10% with placebo. Treatment-emergent grade 3-4 adverse events occurred in 59% receiving pembrolizumab and 38% of patients receiving placebo. Progression-free survival was significantly longer with maintenance pembrolizumab versus placebo (5.4 months [95% CI, 3.1 to 7.3 months] v 3.0 months [95% CI; 2.7 to 5.5 months]; hazard ratio, 0.65; log-rank P = .04; maximum efficiency robust test P = .039). Median overall survival was 22 months (95% CI, 12.9 months to not reached) with pembrolizumab and 18.7 months (95% CI, 11.4 months to not reached) with placebo. There was no significant interaction between PD-L1 CPS ≥ 10 and treatment arm for progression-free survival or overall survival.
Conclusion: Switch maintenance pembrolizumab leads to additional objective responses in patients achieving at least stable disease with first-line platinum-based chemotherapy and prolongs progression-free survival in patients with metastatic urothelial cancer.
Trial registration: ClinicalTrials.gov NCT02500121.
Figures
References
-
- von der Maase H, Sengelov L, Roberts JT, et al: Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol 23:4602-4608, 2005. - PubMed
-
- Galsky MD, Hahn NM, Rosenberg J, et al: Treatment of patients with metastatic urothelial cancer “unfit” for cisplatin-based chemotherapy. J Clin Oncol 29:2432-2438, 2011. - PubMed
-
- von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: Results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;18:3068–3077. - PubMed
-
- Grivas PD, Daignault S, Tagawa ST, et al. Double-blind, randomized, phase 2 trial of maintenance sunitinib versus placebo after response to chemotherapy in patients with advanced urothelial carcinoma. Cancer. 2014;120:692–701. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

