Hematopoiesis and Cardiovascular Disease
- PMID: 32271679
- PMCID: PMC7153537
- DOI: 10.1161/CIRCRESAHA.120.315895
Hematopoiesis and Cardiovascular Disease
Abstract
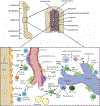
A central feature of atherosclerosis, the most prevalent chronic vascular disease and root cause of myocardial infarction and stroke, is leukocyte accumulation in the arterial wall. These crucial immune cells are produced in specialized niches in the bone marrow, where a complex cell network orchestrates their production and release. A growing body of clinical studies has documented a correlation between leukocyte numbers and cardiovascular disease risk. Understanding how leukocytes are produced and how they contribute to atherosclerosis and its complications is, therefore, critical to understanding and treating the disease. In this review, we focus on the key cells and products that regulate hematopoiesis under homeostatic conditions, during atherosclerosis and after myocardial infarction.
Keywords: atherosclerosis; hematopoiesis; leukocytes; myocardial infarction; stem cell.
Figures



References
-
- World Health Organization. Cardiovascular diseases (CVDs) Fact Sheet. 2017.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

