Diversity of peripheral blood human NK cells identified by single-cell RNA sequencing
- PMID: 32271902
- PMCID: PMC7160259
- DOI: 10.1182/bloodadvances.2019000699
Diversity of peripheral blood human NK cells identified by single-cell RNA sequencing
Abstract
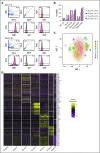
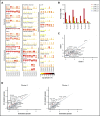
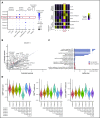
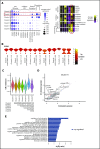
Human natural killer (NK) cells in peripheral blood perform many functions, and classification of specific subsets has been a longstanding goal. We report single-cell RNA sequencing of NK cells, comparing gene expression in unstimulated and interleukin (IL)-2-activated cells from healthy cytomegalovirus (CMV)-negative donors. Three NK cell subsets resembled well-described populations; CD56brightCD16-, CD56dimCD16+CD57-, and CD56dimCD16+CD57+. CD56dimCD16+CD57- cells subdivided to include a population with higher chemokine mRNA and increased frequency of killer-cell immunoglobulin-like receptor expression. Three novel human blood NK cell populations were identified: a population of type I interferon-responding NK cells that were CD56neg; a population exhibiting a cytokine-induced memory-like phenotype, including increased granzyme B mRNA in response to IL-2; and finally, a small population, with low ribosomal expression, downregulation of oxidative phosphorylation, and high levels of immediate early response genes indicative of cellular activation. Analysis of CMV+ donors established that CMV altered the proportion of NK cells in each subset, especially an increase in adaptive NK cells, as well as gene regulation within each subset. Together, these data establish an unexpected diversity in blood NK cells and provide a new framework for analyzing NK cell responses in health and disease.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures






References
-
- Chiossone L, Dumas PY, Vienne M, Vivier E. Natural killer cells and other innate lymphoid cells in cancer [published correction appears in Nat Rev Innunol. 2018;18:726]. Nat Rev Immunol. 2018;18(11):671-688. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

