Circuitry Underlying Experience-Dependent Plasticity in the Mouse Visual System
- PMID: 32272065
- PMCID: PMC7251959
- DOI: 10.1016/j.neuron.2020.01.031
Circuitry Underlying Experience-Dependent Plasticity in the Mouse Visual System
Erratum in
-
Circuitry Underlying Experience-Dependent Plasticity in the Mouse Visual System.Neuron. 2020 Sep 9;107(5):986-987. doi: 10.1016/j.neuron.2020.08.004. Neuron. 2020. PMID: 32910891 Free PMC article. No abstract available.
Abstract
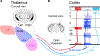
Since the discovery of ocular dominance plasticity, neuroscientists have understood that changes in visual experience during a discrete developmental time, the critical period, trigger robust changes in the visual cortex. State-of-the-art tools used to probe connectivity with cell-type-specific resolution have expanded the understanding of circuit changes underlying experience-dependent plasticity. Here, we review the visual circuitry of the mouse, describing projections from retina to thalamus, between thalamus and cortex, and within cortex. We discuss how visual circuit development leads to precise connectivity and identify synaptic loci, which can be altered by activity or experience. Plasticity extends to visual features beyond ocular dominance, involving subcortical and cortical regions, and connections between cortical inhibitory interneurons. Experience-dependent plasticity contributes to the alignment of networks spanning retina to thalamus to cortex. Disruption of this plasticity may underlie aberrant sensory processing in some neurodevelopmental disorders.
Keywords: binocular matching; cortical circuits; critical periods; inhibitory neurons; neurodevelopmental disorders; receptive field development; sensitive periods; sensory processing; synaptic plasticity; thalamus.
Copyright © 2020 Elsevier Inc. All rights reserved.
Figures


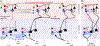
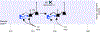
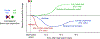
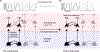

References
-
- Antonini A, and Stryker MP (1993). Rapid remodeling of axonal arbors in the visual cortex. Science 260, 1819–1821. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

