Skeletal muscle in healthy humans exhibits a day-night rhythm in lipid metabolism
- PMID: 32272236
- PMCID: PMC7217992
- DOI: 10.1016/j.molmet.2020.100989
Skeletal muscle in healthy humans exhibits a day-night rhythm in lipid metabolism
Abstract
Objective: Human energy metabolism is under the regulation of the molecular circadian clock; we recently reported that mitochondrial respiration displays a day-night rhythm under study conditions that are similar to real life. Mitochondria are interconnected with lipid droplets, which are of importance in fuel utilization and play a role in muscle insulin sensitivity. Here, we investigated if skeletal muscle lipid content and composition also display day-night rhythmicity in healthy, lean volunteers.
Methods: Skeletal muscle biopsies were obtained from 12 healthy lean male volunteers every 5 h over a 24 h period. Volunteers were provided with standardized meals, and biopsies were taken 4.5 h after each last meal. Lipid droplet size and number were investigated by confocal microscopy. Additionally, the muscle lipidome was assessed using UPLC/HRMS-based semi-targeted lipidomics.
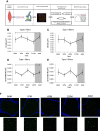
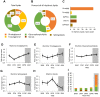
Results: Confocal microscopy revealed diurnal differences in intramyocellular lipid content (P < 0.05) and lipid droplet size in oxidative type 1 muscle fibers (P < 0.01). Lipidomics analysis revealed that 13% of all detected lipids displayed significant day-night rhythmicity. The most rhythmic lipid species were glycerophospholipids and diacylglycerols (DAG), with the latter being the largest fraction (>50% of all rhythmic species). DAG levels showed a day-night pattern with a trough at 1 PM and a peak at 4 AM.
Conclusions: Using two distinct methods, our findings show that myocellular lipid content and whole muscle lipid composition vary across the day-night cycle under normal living conditions. In particular, day-night rhythmicity was present in over half of the DAG lipid species. Future studies are needed to investigate whether rhythmicity in DAG is functionally related to insulin sensitivity and how this might be altered in prediabetes.
Keywords: Circadian clock; Human skeletal muscle; Lipid metabolism; Lipidomics.
Copyright © 2020 The Authors. Published by Elsevier GmbH.. All rights reserved.
Figures

References
-
- Neufeld-Cohen A., Robles M.S., Aviram R., Manella G., Adamovich Y., Ladeuix B. Circadian control of oscillations in mitochondrial rate-limiting enzymes and nutrient utilization by PERIOD proteins. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(12):E1673–E1682. doi: 10.1073/pnas.1519650113. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

