Influence of Carboxylate Anions on Phase Behavior of Choline Ionic Liquid Mixtures
- PMID: 32272688
- PMCID: PMC7180831
- DOI: 10.3390/molecules25071691
Influence of Carboxylate Anions on Phase Behavior of Choline Ionic Liquid Mixtures
Abstract
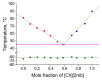
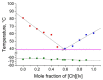
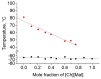
Mixing ionic liquids is a suitable strategy to tailor properties, e.g., to reduce melting points. The present study aims to widen the application range of low-toxic choline-based ionic liquids by studying eight binary phase diagrams of six different choline carboxylates. Five of them show eutectic points with melting points dropping by 13 to 45 °C. The eutectic mixtures of choline acetate and choline 2-methylbutarate were found to melt at 45 °C, which represents a remarkable melting point depression compared to the pure compounds with melting points of 81 (choline acetate) and 90 °C (choline 2-methylbutarate), respectively. Besides melting points, the thermal stabilities of the choline salt mixtures were investigated to define the thermal operation range for potential practical applications of these mixtures. Typical decomposition temperatures were found between 165 and 207 °C, with choline lactate exhibiting the highest thermal stability.
Keywords: choline; differential scanning calorimetry; eutectic mixture; ionic liquid; phase diagram.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



References
-
- Peng H., Wang S., Xu H., Dai G. Preparations, properties, and formation mechanism of novel cellulose hydrogel membrane based on ionic liquid. J. Appl. Polym. Sci. 2018;135:45488. doi: 10.1002/app.45488. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

