The endless frontier? The recent increase of R&D productivity in pharmaceuticals
- PMID: 32272953
- PMCID: PMC7147016
- DOI: 10.1186/s12967-020-02313-z
The endless frontier? The recent increase of R&D productivity in pharmaceuticals
Abstract
Background: Studies on the early 2000s documented increasing attrition rates and duration of clinical trials, leading to a representation of a "productivity crisis" in pharmaceutical research and development (R&D). In this paper, we produce a new set of analyses for the last decade and report a recent increase of R&D productivity within the industry.
Methods: We use an extensive data set on the development history of more than 50,000 projects between 1990 and 2017, which we integrate with data on sales, patents, and anagraphical information on each institution involved. We devise an indicator to quantify the novelty of each project, based on its set of mechanisms of action.
Results: First, we investigate how R&D projects are allocated across therapeutic areas and find a polarization towards high uncertainty/high potential reward indications, with a strong focus on oncology. Second, we find that attrition rates have been decreasing at all stages of clinical research in recent years. In parallel, for each phase, we observe a significant reduction of time required to identify projects to be discontinued. Moreover, our analysis shows that more recent successful R&D projects are increasingly based on novel mechanisms of action and target novel indications, which are characterized by relatively small patient populations. Third, we find that the number of R&D projects on advanced therapies is also growing. Finally, we investigate the relative contribution to productivity variations of different types of institutions along the drug development process, with a specific focus on the distinction between the roles of Originators and Developers of R&D projects. We document that in the last decade Originator-Developer collaborations in which biotech companies act as Developers have been growing in importance. Moreover, we show that biotechnology companies have reached levels of productivity in project development that are equivalent to those of large pharmaceutical companies.
Conclusions: Our study reports on the state of R&D productivity in the bio-pharmaceutical industry, finding several signals of an improving performance, with R&D projects becoming more targeted and novel in terms of indications and mechanisms of action.
Keywords: Attrition rates; Pharmaceutical innovation; R&D productivity.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



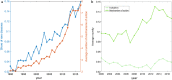
References
-
- Bush V. Science, the endless frontier: a report to the president. Washington, D. C.: U.S. Government Printing Office; 1945.
-
- Gambardella A. Science and innovation: the US pharmaceutical industry during the 1980s. Cambridge: Cambridge University Press; 1995.
-
- McKelvey M, Orsenigo L, Pammolli F. Pharmaceuticals analyzed through the lens of a sectoral innovation system. In: Malerba F, editor. Sectoral systems of innovation: concepts, issues and analyses of six major sectors in Europe. Milan: Bocconi University; 2004. pp. 73–120.

