Neoadjuvant Gemcitabine-Cisplatin Plus Radical Cystectomy-Pelvic Lymph Node Dissection for Muscle-invasive Bladder Cancer: A 12-year Experience
- PMID: 32273235
- PMCID: PMC8375301
- DOI: 10.1016/j.clgc.2020.02.014
Neoadjuvant Gemcitabine-Cisplatin Plus Radical Cystectomy-Pelvic Lymph Node Dissection for Muscle-invasive Bladder Cancer: A 12-year Experience
Abstract
Introduction: The aim of this study was to determine drug delivery/toxicity, and pathologic/surgical outcomes of patients with muscle-invasive bladder cancer (MIBC) receiving neoadjuvant gemcitabine-cisplatin (GC) plus radical cystectomy-pelvic lymph node dissection (RC-PLND).
Patients and methods: Chemotherapy and surgical/pathologic outcomes were retrospectively analyzed with 5-year survival follow-up at a referral center. Post-neoadjuvant chemotherapy (NAC) pathologic endpoints included complete response (pT0N0), residual non-MIBC (pTa/Tis/T1N0), and ≥ MIBC (≥ pT2 and/or N+). Associations of pathologic/surgical findings with overall survival (OS), disease-free survival (DFS), and surgical management with RC-PLND were analyzed (Cox regression).
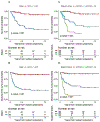
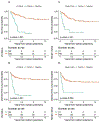
Results: Clinical T2a-T4aN0M0 MIBC patients (n = 154) from January 2000-October 2012 received GC plus RC-PLND. Patients (n = 117; 76%) received GC × 4 and 136 (88%) GC × 3. Five-year OS was 61% (95% confidence interval [CI], 53-71). Median number of resected lymph nodes (LNs) was 19. Down-staging was observed as follows: pT0N0: 21%; pTa/Tis/T1N0: 25%, with similar 5-year OS (85% and 89%, respectively). Five-year OS for < pT2 versus ≥ pT2 residual disease was 87% (95% CI, 78%-98%) versus 38% (95% CI, 27%-53%); P < .001. Post-NAC stage ≥ pT2 (HR, 6.79; 95% CI, 2.63-17.53; P < .001), positive LN (HR, 3.64; 95% CI, 1.84-7.19; P < .001), and positive margins (HR, 4.15; 95% CI, 1.68-10.25; P = .002) were associated with increased risk of all-cause death (multivariable analysis). An HR of 0.97 (95% CI, 0.94-1.00) was observed for each additional node removed, but this effect was not statistically significant (P = .056).
Conclusions: Neoadjuvant GC achieves meaningful pathologic responses. Patients with ≥ pT2 residual disease, positive margins, or positive LN post-chemotherapy have inferior survival.
Keywords: Bladder cancer; Complete pathologic response; Neoadjuvant chemotherapy; Pathologic downstaging; Urothelial carcinoma.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosures:
No relevant conflicts of interest exist for the authors of this manuscript.
Figures

Comment in
-
Urological Oncology: Bladder, Penis and Urethral Cancer, and Basic Principles of Oncology.J Urol. 2022 Aug;208(2):474-476. doi: 10.1097/JU.0000000000002755. Epub 2022 May 20. J Urol. 2022. PMID: 35593062 No abstract available.
References
-
- Stein JP, Lieskovsky G, Cote R, Groshen S, Feng AC, Boyd S, et al.Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. 2001;19(3):666–75. - PubMed
-
- Grossman HB, Natale RB, Tangen CM, Speights VO, Vogelzang NJ, Trump DL, et al.Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349(9):859–66. - PubMed
-
- Advanced Bladder Cancer Meta-analysis C. Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur Urol. 2005;48(2):202–5; discussion 5–6. - PubMed
-
- International Collaboration of T, Medical Research Council Advanced Bladder Cancer Working P, European Organisation for R, Treatment of Cancer Genito-Urinary Tract Cancer G, Australian Bladder Cancer Study G, National Cancer Institute of Canada Clinical Trials G, et al. International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. J Clin Oncol. 2011;29(16):2171–7. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

