Neuronal pentraxin 2: a synapse-derived CSF biomarker in genetic frontotemporal dementia
- PMID: 32273328
- PMCID: PMC7279197
- DOI: 10.1136/jnnp-2019-322493
Neuronal pentraxin 2: a synapse-derived CSF biomarker in genetic frontotemporal dementia
Abstract
Introduction: Synapse dysfunction is emerging as an early pathological event in frontotemporal dementia (FTD), however biomarkers are lacking. We aimed to investigate the value of cerebrospinal fluid (CSF) neuronal pentraxins (NPTXs), a family of proteins involved in homeostatic synapse plasticity, as novel biomarkers in genetic FTD.
Methods: We included 106 presymptomatic and 54 symptomatic carriers of a pathogenic mutation in GRN, C9orf72 or MAPT, and 70 healthy non-carriers participating in the Genetic Frontotemporal dementia Initiative (GENFI), all of whom had at least one CSF sample. We measured CSF concentrations of NPTX2 using an in-house ELISA, and NPTX1 and NPTX receptor (NPTXR) by Western blot. We correlated NPTX2 with corresponding clinical and neuroimaging datasets as well as with CSF neurofilament light chain (NfL) using linear regression analyses.
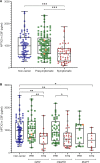
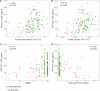
Results: Symptomatic mutation carriers had lower NPTX2 concentrations (median 643 pg/mL, IQR (301-872)) than presymptomatic carriers (1003 pg/mL (624-1358), p<0.001) and non-carriers (990 pg/mL (597-1373), p<0.001) (corrected for age). Similar results were found for NPTX1 and NPTXR. Among mutation carriers, NPTX2 concentration correlated with several clinical disease severity measures, NfL and grey matter volume of the frontal, temporal and parietal lobes, insula and whole brain. NPTX2 predicted subsequent decline in phonemic verbal fluency and Clinical Dementia Rating scale plus FTD modules. In longitudinal CSF samples, available in 13 subjects, NPTX2 decreased around symptom onset and in the symptomatic stage.
Discussion: We conclude that NPTX2 is a promising synapse-derived disease progression biomarker in genetic FTD.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
