Serum neuronal exosomes predict and differentiate Parkinson's disease from atypical parkinsonism
- PMID: 32273329
- PMCID: PMC7361010
- DOI: 10.1136/jnnp-2019-322588
Serum neuronal exosomes predict and differentiate Parkinson's disease from atypical parkinsonism
Abstract
Objective: Parkinson's disease is characterised neuropathologically by α-synuclein aggregation. Currently, there is no blood test to predict the underlying pathology or distinguish Parkinson's from atypical parkinsonian syndromes. We assessed the clinical utility of serum neuronal exosomes as biomarkers across the spectrum of Parkinson's disease, multiple system atrophy and other proteinopathies.
Methods: We performed a cross-sectional study of 664 serum samples from the Oxford, Kiel and Brescia cohorts consisting of individuals with rapid eye movement sleep behavioural disorder, Parkinson's disease, dementia with Lewy bodies, multiple system atrophy, frontotemporal dementia, progressive supranuclear palsy, corticobasal syndrome and controls. Longitudinal samples were analysed from Parkinson's and control individuals. We developed poly(carboxybetaine-methacrylate) coated beads to isolate L1 cell adhesion molecule (L1CAM)-positive extracellular vesicles with characteristics of exosomes and used mass spectrometry or multiplexed electrochemiluminescence to measure exosomal proteins.
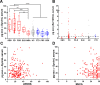
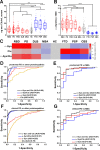
Results: Mean neuron-derived exosomal α-synuclein was increased by twofold in prodromal and clinical Parkinson's disease when compared with multiple system atrophy, controls or other neurodegenerative diseases. With 314 subjects in the training group and 105 in the validation group, exosomal α-synuclein exhibited a consistent performance (AUC=0.86) in separating clinical Parkinson's disease from controls across populations. Exosomal clusterin was elevated in subjects with non-α-synuclein proteinopathies. Combined neuron-derived exosomal α-synuclein and clusterin measurement predicted Parkinson's disease from other proteinopathies with AUC=0.98 and from multiple system atrophy with AUC=0.94. Longitudinal sample analysis showed that exosomal α-synuclein remains stably elevated with Parkinson's disease progression.
Conclusions: Increased α-synuclein egress in serum neuronal exosomes precedes the diagnosis of Parkinson's disease, persists with disease progression and in combination with clusterin predicts and differentiates Parkinson's disease from atypical parkinsonism.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: HW and JR are employees of Eli Lilly and Company.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
