PSGL-1 restricts HIV-1 infectivity by blocking virus particle attachment to target cells
- PMID: 32273392
- PMCID: PMC7196789
- DOI: 10.1073/pnas.1916054117
PSGL-1 restricts HIV-1 infectivity by blocking virus particle attachment to target cells
Abstract
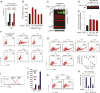
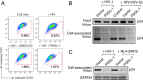
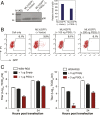
P-selectin glycoprotein ligand-1 (PSGL-1) is a dimeric, mucin-like, 120-kDa glycoprotein that binds to P-, E-, and L-selectins. PSGL-1 is expressed primarily on the surface of lymphoid and myeloid cells and is up-regulated during inflammation to mediate leukocyte tethering and rolling on the surface of endothelium for migration into inflamed tissues. Although it has been reported that PSGL-1 expression inhibits HIV-1 replication, the mechanism of PSGL-1-mediated anti-HIV activity remains to be elucidated. Here we report that PSGL-1 in virions blocks the infectivity of HIV-1 particles by preventing the binding of particles to target cells. This inhibitory activity is independent of the viral glycoprotein present on the virus particle; the binding of particles bearing the HIV-1 envelope glycoprotein or vesicular stomatitis virus G glycoprotein or even lacking a viral glycoprotein is impaired by PSGL-1. Mapping studies show that the extracellular N-terminal domain of PSGL-1 is necessary for its anti-HIV-1 activity, and that the PSGL-1 cytoplasmic tail contributes to inhibition. In addition, we demonstrate that the PSGL-1-related monomeric E-selectin-binding glycoprotein CD43 also effectively blocks HIV-1 infectivity. HIV-1 infection, or expression of either Vpu or Nef, down-regulates PSGL-1 from the cell surface; expression of Vpu appears to be primarily responsible for enabling the virus to partially escape PSGL-1-mediated restriction. Finally, we show that PSGL-1 inhibits the infectivity of other viruses, such as murine leukemia virus and influenza A virus. These findings demonstrate that PSGL-1 is a broad-spectrum antiviral host factor with a unique mechanism of action.
Keywords: CD43; HIV-1; Nef; PSGL-1; Vpu.
Copyright © 2020 the Author(s). Published by PNAS.
Conflict of interest statement
Competing interest statement: Two provisional patent applications pertaining to the results presented in this paper have been filed by George Mason University.
Figures


References
-
- Sako D., et al. , Expression cloning of a functional glycoprotein ligand for P-selectin. Cell 75, 1179–1186 (1993). - PubMed
-
- Guyer D. A., et al. , P-selectin glycoprotein ligand-1 (PSGL-1) is a ligand for L-selectin in neutrophil aggregation. Blood 88, 2415–2421 (1996). - PubMed
-
- Laszik Z., et al. , P-selectin glycoprotein ligand-1 is broadly expressed in cells of myeloid, lymphoid, and dendritic lineage and in some nonhematopoietic cells. Blood 88, 3010–3021 (1996). - PubMed
-
- Fujimoto T. T., et al. , Expression and functional characterization of the P-selectin glycoprotein ligand-1 in various cells. Int. J. Hematol. 64, 231–239 (1996). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

