Ocrelizumab initiation in patients with MS: A multicenter observational study
- PMID: 32273482
- PMCID: PMC7176249
- DOI: 10.1212/NXI.0000000000000719
Ocrelizumab initiation in patients with MS: A multicenter observational study
Abstract
Objective: To provide first real-world experience on patients with MS treated with the B cell-depleting antibody ocrelizumab.
Methods: We retrospectively collected data of patients who had received at least 1 treatment cycle (2 infusions) of ocrelizumab at 3 large neurology centers. Patients' characteristics including premedication, clinical disease course, and documented side effects were analyzed.
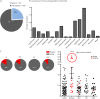
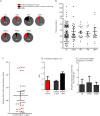
Results: We could identify 210 patients (125 women, mean age ± SD, 42.1 ± 11.4 years) who had received ocrelizumab with a mean disease duration of 7.3 years and a median Expanded Disability Status Scale score of 3.75 (interquartile range 2.5-5.5; range 0-8). Twenty-six percent of these patients had a primary progressive MS (PPMS), whereas 74% had a relapsing-remitting (RRMS) or active secondary progressive (aSPMS) disease course. Twenty-four percent of all patients were treatment naive, whereas 76% had received immune therapies before. After ocrelizumab initiation (median follow-up was 200 days, range 30-1,674 days), 13% of patients with RRMS/aSPMS experienced a relapse (accounting for an annualized relapse rate of 0.17, 95% CI 0.10-0.24), and 5% of all patients with MS experienced a 12-week confirmed disability progression. Treatment was generally well tolerated, albeit only short-term side effects were recorded, including direct infusion-related reactions and mild infections.
Conclusions: We provide class IV evidence that treatment with ocrelizumab can stabilize naive and pretreated patients, indicating that ocrelizumab is an option following potent MS drugs such as natalizumab and fingolimod. Further studies are warranted to confirm these findings and to reveal safety concerns in the longer-term follow-up.
Classification of evidence: This study provides Class IV evidence that for patients with MS, ocrelizumab can stabilize both treatment-naive and previously treated patients.
Copyright © 2020 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures
References
-
- Hauser SL, Bar-Or A, Comi G, et al. . Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med 2017;376:221–234. - PubMed
-
- Montalban X, Hauser SL, Kappos L, et al. . Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med 2017;376:209–220. - PubMed
-
- Menge T, Dubey D, Warnke C, Hartung HP, Stüve O. Ocrelizumab for the treatment of relapsing-remitting multiple sclerosis. Expert Rev Neurother 2016;16:1131–1139. - PubMed
-
- Hauser SL, Waubant E, Arnold DL, et al. . B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med 2008;358:676–688. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
