Differential Role for a Defined Lateral Horn Neuron Subset in Naïve Odor Valence in Drosophila
- PMID: 32273557
- PMCID: PMC7145822
- DOI: 10.1038/s41598-020-63169-3
Differential Role for a Defined Lateral Horn Neuron Subset in Naïve Odor Valence in Drosophila
Abstract
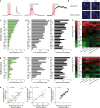
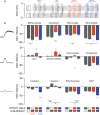
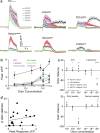
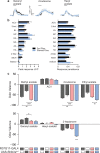
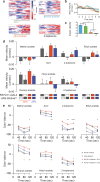
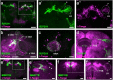
Value coding of external stimuli in general, and odor valence in particular, is crucial for survival. In flies, odor valence is thought to be coded by two types of neurons: mushroom body output neurons (MBONs) and lateral horn (LH) neurons. MBONs are classified as neurons that promote either attraction or aversion, but not both, and they are dynamically activated by upstream neurons. This dynamic activation updates the valence values. In contrast, LH neurons receive scaled, but non-dynamic, input from their upstream neurons. It remains unclear how such a non-dynamic system generates differential valence values. Recently, PD2a1/b1 LH neurons were demonstrated to promote approach behavior at low odor concentration in starved flies. Here, we demonstrate that at high odor concentrations, these same neurons contribute to avoidance in satiated flies. The contribution of PD2a1/b1 LH neurons to aversion is context dependent. It is diminished in starved flies, although PD2a1/b1 neural activity remains unchanged, and at lower odor concentration. In addition, PD2a1/b1 aversive effect develops over time. Thus, our results indicate that, even though PD2a1/b1 LH neurons transmit hard-wired output, their effect on valence can change. Taken together, we suggest that the valence model described for MBONs does not hold for LH neurons.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

