Poly(N-isopropylacrylamide) based thin microgel films for use in cell culture applications
- PMID: 32273560
- PMCID: PMC7145875
- DOI: 10.1038/s41598-020-63228-9
Poly(N-isopropylacrylamide) based thin microgel films for use in cell culture applications
Abstract
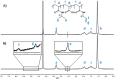
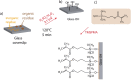
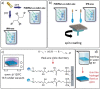
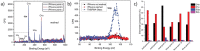
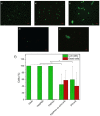
Poly(N-isopropylacrylamide) (PNIPAm) is widely used to fabricate cell sheet surfaces for cell culturing, however copolymer and interpenetrated polymer networks based on PNIPAm have been rarely explored in the context of tissue engineering. Many complex and expensive techniques have been employed to produce PNIPAm-based films for cell culturing. Among them, spin coating has demonstrated to be a rapid fabrication process of thin layers with high reproducibility and uniformity. In this study, we introduce an innovative approach to produce anchored smart thin films both thermo- and electro-responsive, with the aim to integrate them in electronic devices and better control or mimic different environments for cells in vitro. Thin films were obtained by spin coating of colloidal solutions made by PNIPAm and PAAc nanogels. Anchoring the films to the substrates was obtained through heat treatment in the presence of dithiol molecules. From analyses carried out with AFM and XPS, the final samples exhibited a flat morphology and high stability to water washing. Viability tests with cells were finally carried out to demonstrate that this approach may represent a promising route to integrate those hydrogels films in electronic platforms for cell culture applications.
Conflict of interest statement
The authors have no competing interests as defined by Nature Research, or other interests that might be perceived to influence the results and/or discussion reported in this paper.
Figures








References
-
- Migliorini L, Santaniello T, Yan Y, Lenardi C, Milani P. Low-voltage electrically driven homeostatic hydrogel-based actuators for underwater soft robotics. Sensors Actuators, B Chem. 2016;228:758–766. doi: 10.1016/j.snb.2016.01.110. - DOI
-
- Leo, D. J. Engineering Analysis of Smart Material Systems. (John Wiley & Sons, Inc.). 10.1002/9780470209721. 2007.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

