Development and Validation of the Modified Patient-Reported Outcome Scale for Chronic Obstructive Pulmonary Disease (mCOPD-PRO)
- PMID: 32273695
- PMCID: PMC7108702
- DOI: 10.2147/COPD.S240842
Development and Validation of the Modified Patient-Reported Outcome Scale for Chronic Obstructive Pulmonary Disease (mCOPD-PRO)
Abstract
Purpose: The present study aimed to develop and validate the modified patient-reported outcome scale for chronic obstructive pulmonary disease (mCOPD-PRO) for measuring the health status in COPD using both classical test theory and item response theory.
Methods: A working group was initially established. The conceptual framework of COPD-PRO was modified. Subsequently, items related to COPD were gathered and selected through expert consultation, patient cognitive interviewing, classical test theory methods, as well as the item response theory method. Finally, the formed mCOPD-PRO was evaluated in terms of reliability, content validity, construct validity, criterion validity, known groups validity, and feasibility.
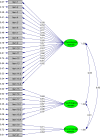
Results: A total of 155 items were gathered in the item bank, and two rounds of expert consultation, interviews with patients and field survey were conducted. The mCOPD-PRO included 27 items in the physiological, psychological, and environmental domains. The Cronbach's alpha of the instrument was 0.954. The correlation coefficients between the scores of each item and its domain scores ranged from 0.429 to 0.902. Confirmatory factor analysis showed that the comparative fit index, incremental fit index, non-normed fit index, standardized root-mean-square residual, and root-mean-square error of approximate were 0.91, 0.91, 0.90, 0.11, and 0.16, respectively. The correlation coefficient between mCOPD-PRO total scores and COPD assessment test scores and the modified Medical Research Council dyspnea scale scores was 0.771 and 0.651, respectively. The differences in mCOPD-PRO total scores and domain scores between the mild/moderate group and severe/extremely severe group of patients with COPD were both statistically significant (P<0.01). The acceptance and completion rates of mCOPD-PRO were both 99.5%, and the median completion time was 5 min (IQR, 4-11 min).
Conclusion: The 27-item mCOPD-PRO is well developed and has good reliability, validity, and feasibility. It may provide a scientific and effective instrument for the clinical evaluation of COPD.
Keywords: chronic obstructive pulmonary disease; classical test theory; instrument; item response theory; patient-reported outcome.
© 2020 Li et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures
Similar articles
-
Psychometric properties of computerized adaptive testing for chronic obstructive pulmonary disease patient-reported outcome measurement.Health Qual Life Outcomes. 2024 Sep 4;22(1):73. doi: 10.1186/s12955-024-02291-6. Health Qual Life Outcomes. 2024. PMID: 39227972 Free PMC article.
-
LC-PROM: Validation of a patient reported outcomes measure for liver cirrhosis patients.Health Qual Life Outcomes. 2016 May 10;14:75. doi: 10.1186/s12955-016-0482-y. Health Qual Life Outcomes. 2016. PMID: 27165036 Free PMC article.
-
Development and validation of a patient reported outcome instrument for chronic obstructive pulmonary diseases.Chin J Integr Med. 2015 Sep;21(9):667-75. doi: 10.1007/s11655-014-1982-4. Epub 2014 Sep 24. Chin J Integr Med. 2015. PMID: 25253553
-
The development of a patient-reported outcome measure for assessing nighttime symptoms of chronic obstructive pulmonary disease.Health Qual Life Outcomes. 2013 Jun 25;11:104. doi: 10.1186/1477-7525-11-104. Health Qual Life Outcomes. 2013. PMID: 23799883 Free PMC article. Review.
-
Development and validation of the patient-reported outcome scale for chronic kidney disease.Int Urol Nephrol. 2024 Feb;56(2):653-665. doi: 10.1007/s11255-023-03702-1. Epub 2023 Jul 15. Int Urol Nephrol. 2024. PMID: 37452989 Free PMC article. Review.
Cited by
-
Development and Validation of a Patient-Reported Outcome Scale for Tension-Type Headache.Front Neurol. 2021 Aug 27;12:693553. doi: 10.3389/fneur.2021.693553. eCollection 2021. Front Neurol. 2021. PMID: 34512514 Free PMC article.
-
The effect of Chronic Care Model-based follow-up on self-efficacy and patient-reported outcomes in COPD patients: a randomized controlled study.BMC Nurs. 2025 May 22;24(1):578. doi: 10.1186/s12912-025-03247-x. BMC Nurs. 2025. PMID: 40405196 Free PMC article.
-
Association of Patient-Reported Outcome Patterns and Major Clinical Factors with Frailty in Stable COPD.Int J Chron Obstruct Pulmon Dis. 2025 Jun 12;20:1927-1937. doi: 10.2147/COPD.S517270. eCollection 2025. Int J Chron Obstruct Pulmon Dis. 2025. PMID: 40529222 Free PMC article.
-
Association of Frailty with Patient-Report Outcomes and Major Clinical Determinants in Patients with Acute Exacerbation of Chronic Obstructive Pulmonary Disease.Int J Chron Obstruct Pulmon Dis. 2024 Apr 12;19:907-919. doi: 10.2147/COPD.S444580. eCollection 2024. Int J Chron Obstruct Pulmon Dis. 2024. PMID: 38628984 Free PMC article.
-
Psychometric properties of computerized adaptive testing for chronic obstructive pulmonary disease patient-reported outcome measurement.Health Qual Life Outcomes. 2024 Sep 4;22(1):73. doi: 10.1186/s12955-024-02291-6. Health Qual Life Outcomes. 2024. PMID: 39227972 Free PMC article.
References
-
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease; 2019. Available from: http://www.goldcopd.org. Accessed November11, 2019. - PubMed
-
- U.S. Food and Drug Administration. Guidance for industry patient-reported outcome measures: use in medical product development to support labeling claims. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents.... Accessed November11, 2019. - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical