Long Non-Coding RNA CASC19 Sponges microRNA-532 and Promotes Oncogenicity of Clear Cell Renal Cell Carcinoma by Increasing ETS1 Expression
- PMID: 32273759
- PMCID: PMC7102911
- DOI: 10.2147/CMAR.S242472
Long Non-Coding RNA CASC19 Sponges microRNA-532 and Promotes Oncogenicity of Clear Cell Renal Cell Carcinoma by Increasing ETS1 Expression
Retraction in
-
Long Non-Coding RNA CASC19 Sponges microRNA-532 and Promotes Oncogenicity of Clear Cell Renal Cell Carcinoma by Increasing ETS1 Expression [Retraction].Cancer Manag Res. 2023 Jul 13;15:683-684. doi: 10.2147/CMAR.S430060. eCollection 2023. Cancer Manag Res. 2023. PMID: 37465079 Free PMC article.
Expression of concern in
-
Long Non-Coding RNA CASC19 Sponges MicroRNA-532 and Promotes Oncogenicity of Clear Cell Renal Cell Carcinoma by Increasing ETS1 Expression [Expression of Concern].Cancer Manag Res. 2022 Aug 4;14:2305-2306. doi: 10.2147/CMAR.S384690. eCollection 2022. Cancer Manag Res. 2022. PMID: 35958952 Free PMC article. No abstract available.
Abstract
Purpose: The long non-coding RNA cancer susceptibility 19 (CASC19) is recognized as an important regulator in gastric cancer, colorectal cancer, and non-small cell lung cancer. Nevertheless, to the best of our knowledge, the expression status and detailed roles of CASC19 in clear cell renal cell carcinoma (ccRCC) have not been elucidated. Hence, we aimed to determine CASC19 expression in ccRCC and investigate its roles in ccRCC oncogenicity. The molecular mechanisms underlying CASC19 functions in ccRCC were also determined.
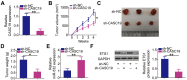
Methods: CASC19 expression was measured by using reverse transcription-quantitative polymerase chain reaction. The effects of CASC19 on ccRCC cell proliferation, colony formation, migration, and invasiveness in vitro, as well as on tumor growth in vivo, were examined by the MTT assay, colony formation assay, cell migration and invasiveness assays, and tumor xenograft in nude nice, respectively.
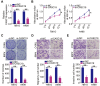
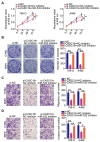
Results: CASC19 was overexpressed in ccRCC tissues and cell lines. High expression of CASC19 was closely associated with unfavorable clinicopathological parameters and predicted negative clinical outcomes in patients with ccRCC. Knockdown of CASC19 decreased ccRCC cell proliferation, colony formation, migration, and invasiveness, as well as attenuated tumor growth in vivo. Mechanistically, CASC19 functioned as a competing endogenous RNA and upregulated the expression of ETS proto-oncogene 1 (ETS1) through sponging microRNA-532 (miR-532). Furthermore, rescue assays revealed that inhibiting miR-532 or restoring ETS1 expression partially abolished the impacts of CASC19 knockdown on ccRCC cells.
Conclusion: The CASC19/miR-532/ETS1 regulatory pathway is crucial for the malignant manifestations of ccRCC, which makes it an attractive target for potential treatments of ccRCC.
Keywords: MTT assay; cancer; cell migration; invasiveness; knockdown; xenograft.
© 2020 Luo et al.
Conflict of interest statement
The authors declare that they have no competing interests in this work.
Figures




References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

