Mite load predicts the quality of sexual color and locomotor performance in a sexually dichromatic lizard
- PMID: 32273977
- PMCID: PMC7141043
- DOI: 10.1002/ece3.5689
Mite load predicts the quality of sexual color and locomotor performance in a sexually dichromatic lizard
Abstract
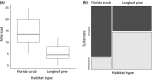
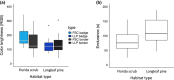
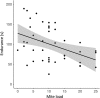
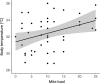
Since Darwin, the maintenance of bright sexual colors has recurrently been linked to mate preference. However, the mechanisms underpinning such preferences for bright colors would not be resolved for another century. Likely, the idea of selection for colors that could decrease the chances of survival (e.g., flashy colors that can inadvertently attract predators) was perceived as counterintuitive. It is now widely accepted that these extreme colors often communicate to mates the ability to survive despite a "handicap" and act as honest signals of individual quality when they are correlated with the quality of other traits that are directly linked to individual fitness. Sexual colors in males are frequently perceived as indicators of infection resistance, in particular. Still, there remains considerable discord among studies attempting to parse the relationships between the variables associating sexual color and infection resistance, such as habitat type and body size. This discord may arise from complex interactions between these variables. Here, we ask if sexual color in male Florida scrub lizards (Sceloporus woodi) is an honest signal of resistance to chigger mite infection. To this end, we use linear modeling to explore relationships between mite load, different components of sexual color, ecological performance, body size, and habitat type. Our data show that that the brightness of sexual color in scrub lizards is negatively associated with the interaction between mite load and body size, and scrub lizards suffer decreased endurance capacity with increases in mite load. Our data also indicate that mite load, performance, and sexual color in male scrub lizards can vary between habitat types. Collectively, these results suggest that sexual color in scrub lizards is an honest indicator of individual quality and further underscore the importance of considering multiple factors when testing hypotheses related to the maintenance of sexual color.
Keywords: honest signals; parasite‐mediated selection; selection for handicap; sexual dichromatism.
© 2019 The Authors. Ecology and Evolution published by John Wiley & Sons Ltd.
Conflict of interest statement
None declared.
Figures


Similar articles
-
Resolving tradeoffs among crypsis, escape behavior, and microhabitat use in sexually dichromatic species.Oecologia. 2019 Jan;189(1):91-104. doi: 10.1007/s00442-018-4301-5. Epub 2018 Nov 14. Oecologia. 2019. PMID: 30430233
-
Sexual dichromatism and color diversity in the spiny lava lizard Tropidurus spinulosus using lizard visual modelling.Sci Rep. 2019 Oct 3;9(1):14270. doi: 10.1038/s41598-019-50712-0. Sci Rep. 2019. PMID: 31582783 Free PMC article.
-
Effects of natural habitat fragmentation on an endemic scrub lizard (Sceloporus woodi): an historical perspective based on a mitochondrial DNA gene genealogy.Mol Ecol. 1999 Jul;8(7):1093-104. doi: 10.1046/j.1365-294x.1999.00653.x. Mol Ecol. 1999. PMID: 10447851
-
Complex plumages spur rapid color diversification in kingfishers (Aves: Alcedinidae).Elife. 2023 Apr 21;12:e83426. doi: 10.7554/eLife.83426. Elife. 2023. PMID: 37083474 Free PMC article.
-
Virulence of lizard malaria: the evolutionary ecology of an ancient parasite-host association.Parasitology. 1990;100 Suppl:S35-52. doi: 10.1017/s0031182000073005. Parasitology. 1990. PMID: 2235062 Review.
Cited by
-
Using Delaunay triangulation to sample whole-specimen color from digital images.Ecol Evol. 2021 Aug 20;11(18):12468-12484. doi: 10.1002/ece3.7992. eCollection 2021 Sep. Ecol Evol. 2021. PMID: 34594513 Free PMC article.
-
Biology, Systematics, Microbiome, Pathogen Transmission and Control of Chiggers (Acari: Trombiculidae, Leeuwenhoekiidae) with Emphasis on the United States.Int J Environ Res Public Health. 2022 Nov 17;19(22):15147. doi: 10.3390/ijerph192215147. Int J Environ Res Public Health. 2022. PMID: 36429867 Free PMC article. Review.
-
Impact of food availability on the thermal performance curves of male European green lizards (Lacerta viridis).Oecologia. 2025 Apr 1;207(4):57. doi: 10.1007/s00442-025-05699-z. Oecologia. 2025. PMID: 40167788 Free PMC article.
References
-
- Andersen, G. N. , Hägglund, M. , Nagaeva, O. , Frängsmyr, L. , Petrovska, R. , Mincheva‐Nilsson, L. , & Wikberg, J. E. S. (2005). Quantitative measurement of the levels of melanocortin receptor subtype 1, 2, 3 and 5 and pro‐opio‐melanocortin peptide gene expression in subsets of human peripheral blood leucocytes. Scandinavian Journal of Immunology, 61(3), 279–284. 10.1111/j.1365-3083.2005.01565.x - DOI - PubMed
-
- Biaggini, M. , Berti, R. , & Corti, C. (2009). Different habitats, different pressures? Analysis of escape behaviour and ectoparasite load in Podarcis sicula (Lacertidae) populations in different agricultural habitats. Amphibia‐Reptilia, 30(4), 453–461. 10.1163/156853809789647068 - DOI
-
- Bishop, S. C. , & Morris, C. A. (2007). Genetics of disease resistance in sheep and goats. Small Ruminant Research, 70(1), 48–59. 10.1016/j.smallrumres.2007.01.006 - DOI
Associated data
LinkOut - more resources
Full Text Sources

