Z-Scheme Photocatalytic Systems for Solar Water Splitting
- PMID: 32274312
- PMCID: PMC7141076
- DOI: 10.1002/advs.201903171
Z-Scheme Photocatalytic Systems for Solar Water Splitting
Abstract
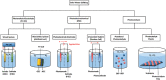
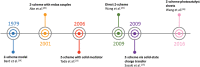
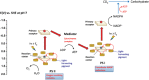
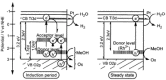
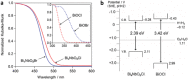
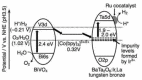
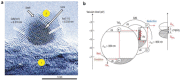
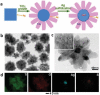
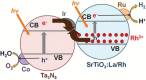
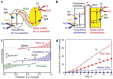
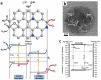
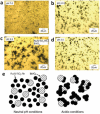
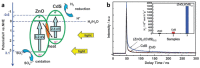
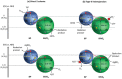
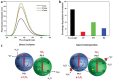
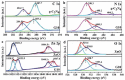
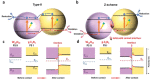
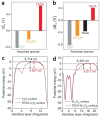
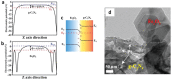
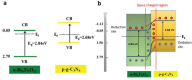
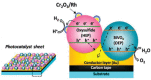
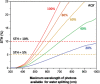
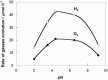
As the world decides on the next giant step for the renewable energy revolution, scientists have begun to reinforce their headlong dives into the exploitation of solar energy. Hitherto, numerous attempts are made to imitate the natural photosynthesis of plants by converting solar energy into chemical fuels which resembles the "Z-scheme" process. A recreation of this system is witnessed in artificial Z-scheme photocatalytic water splitting to generate hydrogen (H2). This work outlines the recent significant implication of the Z-scheme system in photocatalytic water splitting, particularly in the role of electron mediator and the key factors that improve the photocatalytic performance. The Review begins with the fundamental rationales in Z-scheme water splitting, followed by a survey on the development roadmap of three different generations of Z-scheme system: 1) PS-A/D-PS (first generation), 2) PS-C-PS (second generation), and 3) PS-PS (third generation). Focus is also placed on the scaling up of the "leaf-to-tree" challenge of Z-scheme water splitting system, which is also known as Z-scheme photocatalyst sheet. A detailed investigation of the Z-scheme system for achieving H2 evolution from past to present accompanied with in-depth discussion on the key challenges in the area of Z-scheme photocatalytic water splitting are provided.
Keywords: Z‐scheme; artificial photosynthesis; electron mediators; hydrogen; water splitting.
© 2020 The Authors. Published by WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





























References
-
- Global B., BP Statistical Review of World Energy 2017. (Online), https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdf... (accessed September 2019).
-
- Irena., Renewable Energy Topic. (Online) https://resourceirena.irena.org/gateway/dashboard/index.html?topic=4&sub... (accessed September 2019).
-
- Kim J. H., Hansora D., Sharma P., Jang J. W., Lee J. S., Chem. Soc. Rev. 2019, 48, 1908. - PubMed
-
- a) Putri L. K., Ng B.‐J., Er C.‐C., Ong W.‐J., Chang W. S., Mohamed A. R., Chai S.‐P., Appl. Surf. Sci. 2020, 504, 144427;
- b) Kong X. Y., Tan W. L., Ng B.‐J., Chai S.‐P., Mohamed A. R., Nano Res. 2017, 10, 1720;
- c) Tang J.‐Y., Kong X. Y., Ng B.‐J., Chew Y.‐H., Mohamed A. R., Chai S.‐P., Catal. Sci. Technol. 2019, 9, 2335.
-
- a) Xu J., Cao X., Chem. Eng. J. 2015, 260, 642;
- b) Putri L. K., Ng B.‐J., Tan K. H., Lim F. S., Ong W.‐J., Chang W. S., Chai S.‐P., Catal. Today 2018, 315, 93.
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
