Role of Sheet-Edge Interactions in β-sheet Self-Assembling Peptide Hydrogels
- PMID: 32275138
- PMCID: PMC7304824
- DOI: 10.1021/acs.biomac.0c00229
Role of Sheet-Edge Interactions in β-sheet Self-Assembling Peptide Hydrogels
Abstract
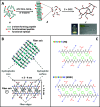
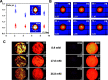
Hydrogels' hydrated fibrillar nature makes them the material of choice for the design and engineering of 3D scaffolds for cell culture, tissue engineering, and drug-delivery applications. One particular class of hydrogels which has been the focus of significant research is self-assembling peptide hydrogels. In the present work, we were interested in exploring how fiber-fiber edge interactions affect the self-assembly and gelation properties of amphipathic peptides. For this purpose, we investigated two β-sheet-forming peptides, FEFKFEFK (F8) and KFEFKFEFKK (KF8K), the latter one having the fiber edges covered by lysine residues. Our results showed that the addition of the two lysine residues did not affect the ability of the peptides to form β-sheet-rich fibers, provided that the overall charge carried by the two peptides was kept constant. However, it did significantly reduce edge-driven hydrophobic fiber-fiber associative interactions, resulting in reduced tendency for KF8K fibers to associate/aggregate laterally and form large fiber bundles and consequently network cross-links. This effect resulted in the formation of hydrogels with lower moduli but faster dynamics. As a result, KF8K fibers could be aligned only under high shear and at high concentration while F8 hydrogel fibers were found to align readily at low shear and low concentration. In addition, F8 hydrogels were found to fragment at high concentration because of the high aggregation state stabilizing the fiber bundles, resulting in fiber breakage rather than disentanglement and alignment.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

