Randomized Phase II Trial of Nivolumab Versus Nivolumab and Ipilimumab for Recurrent or Persistent Ovarian Cancer: An NRG Oncology Study
- PMID: 32275468
- PMCID: PMC7255977
- DOI: 10.1200/JCO.19.02059
Randomized Phase II Trial of Nivolumab Versus Nivolumab and Ipilimumab for Recurrent or Persistent Ovarian Cancer: An NRG Oncology Study
Erratum in
-
Errata.J Clin Oncol. 2020 Aug 10;38(23):2702. doi: 10.1200/JCO.20.01943. J Clin Oncol. 2020. PMID: 32755509 Free PMC article. No abstract available.
Abstract
Purpose: Single-agent PD-1 blockade exhibits limited efficacy in epithelial ovarian cancer (EOC). We evaluated ipilimumab plus nivolumab compared with nivolumab alone in women with persistent or recurrent EOC.
Methods: Eligibility criteria included measurable disease, 1-3 prior regimens, and platinum-free interval (PFI) < 12 months. Participants were randomly allocated to intravenous nivolumab (every 2 weeks) or induction with nivolumab plus ipilimumab for 4 doses (every 3 weeks), followed by every-2-week maintenance nivolumab for a maximum of 42 doses. The primary null hypothesis was equal probability of objective response within 6 months of random allocation in each arm.
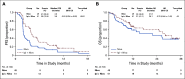
Results: One hundred patients were allocated to receive either nivolumab (n = 49), or nivolumab plus ipilimumab (n = 51), with PFI of < 6 months in 62%. Six (12.2%) responses occurred within 6 months in the nivolumab group and 16 (31.4%) in the nivolumab plus ipilimumab group (odds ratio, 3.28; 85% CI, 1.54 to infinity; P = .034). The median progression-free survival (PFS) was 2 and 3.9 months in the nivolumab and nivolumab plus ipilimumab groups, respectively, with a PFI-stratified hazard ratio of 0.53 (95% CI, 0.34 to 0.82); the respective hazard ratio for death was 0.79 (95% CI, 0.44 to 1.42). Grade ≥ 3 related adverse events occurred in 33% of patients in the nivolumab group and 49% in the combination group, with no treatment-related deaths. PD-L1 expression was not significantly associated with response in either treatment group.
Conclusion: Compared with nivolumab alone, the combination of nivolumab and ipilimumab in EOC resulted in superior response rate and longer, albeit limited, PFS, with toxicity of the combination regimen comparable to prior reports. Additional combination studies to enhance durability of the dual regimen are warranted.
Figures



References
-
- Zhang L, Conejo-Garcia JR, Katsaros D, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–213. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

