Boron doped silver-copper alloy nanoparticle targeting intracellular S. aureus in bone cells
- PMID: 32275737
- PMCID: PMC7147743
- DOI: 10.1371/journal.pone.0231276
Boron doped silver-copper alloy nanoparticle targeting intracellular S. aureus in bone cells
Abstract
Objectives: Alloyed metallic nanoparticles of silver and copper are effective against intracellular infection. However, systemic toxicity may arise due to the non-specific delivery of the nanoparticles. In addressing the issue, this study deals with the targeting of silver-copper-boron (ACB) nanoparticles to infected osteoblasts, which could decrease systemic toxicity and form the basis of targeting specific markers expressed in bone infections.
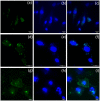
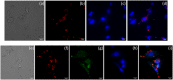
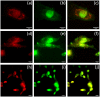
Methods: ACB nanoparticles were synthesized and conjugated to the Cadherin-11 antibody (OBAb). The effect of targeting nanoparticles against extracellular and intracellular S. aureus was determined by enumeration of bacterial growth. The binding of the targeting nanoparticles to infected osteoblasts as well as the visualization of live/dead bacteria due to treatment was carried out using fluorescence microscopy. MTT assay was used to determine the viability of osteoblasts with different concentrations of the nanoparticles.
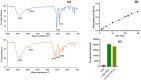
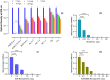
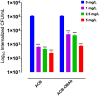
Results: The ACB nanoparticles conjugated to OBAb (ACB-OBAb) were effective against extracellular S. aureus. The ACB-OBAb nanoparticles showed a 1.32 log reduction of intracellular S. aureus at a concentration of 1mg/L. The ACB-OBAb nanoparticles were able to bind to the infected osteoblast and showed toxicity to osteoblasts at levels ≥20mg/L. Also, the percentage of silver, copper, and boron in the nanoparticles determined the effectiveness of their antibacterial activity.
Conclusion: The ACB-OBAb nanoparticles were able to target the osteoblasts and demonstrated significant antibacterial activity against intracellular S. aureus. Targeting shows promise as a strategy to target specific markers expressed on infected osteoblasts for efficient nanoparticle delivery, and further animal studies are recommended to test its efficacy in vivo.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Kavanagh N, Ryan EJ, Widaa A, Sexton G, Fennell J, O’Rourke S, et al. Staphylococcal Osteomyelitis: Disease Progression, Treatment Challenges, and Future Directions. Clin Microbiol Rev [Internet]. 2018. April 1 [cited 2018 Oct 2];31(2):e00084–17. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29444953 - PMC - PubMed
-
- Mathews CJ, Weston VC, Jones A, Field M, Coakley G. Bacterial septic arthritis in adults. Vol. 375, The Lancet. 2010. p. 846–55. - PubMed
-
- Ross KM, Mehr JS, Carothers BL, Greeley RD, Benowitz I, Henry D, et al. Bacterial septic arthritis infections associated with intra-articular injection practices for osteoarthritis knee pain—New Jersey, 2017. Infect Control Hosp Epidemiol. 2019. September 1;40(9):1013–8. 10.1017/ice.2019.168 - DOI - PMC - PubMed
-
- Kang J, Dietz MJ, Hughes K, Xing M, Li B. Silver nanoparticles present high intracellular and extracellular killing against Staphylococcus aureus. J Antimicrob Chemother [Internet]. 2019. June 1 [cited 2019 Dec 19];74(6):1578–85. Available from: https://academic.oup.com/jac/article/74/6/1578/5333167 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

