Deep residual learning for neuroimaging: An application to predict progression to Alzheimer's disease
- PMID: 32275915
- PMCID: PMC7297044
- DOI: 10.1016/j.jneumeth.2020.108701
Deep residual learning for neuroimaging: An application to predict progression to Alzheimer's disease
Abstract
Background: The unparalleled performance of deep learning approaches in generic image processing has motivated its extension to neuroimaging data. These approaches learn abstract neuroanatomical and functional brain alterations that could enable exceptional performance in classification of brain disorders, predicting disease progression, and localizing brain abnormalities.
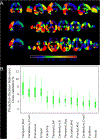
New method: This work investigates the suitability of a modified form of deep residual neural networks (ResNet) for studying neuroimaging data in the specific application of predicting progression from mild cognitive impairment (MCI) to Alzheimer's disease (AD). Prediction was conducted first by training the deep models using MCI individuals only, followed by a domain transfer learning version that additionally trained on AD and controls. We also demonstrate a network occlusion based method to localize abnormalities.
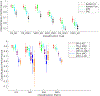
Results: The implemented framework captured non-linear features that successfully predicted AD progression and also conformed to the spectrum of various clinical scores. In a repeated cross-validated setup, the learnt predictive models showed highly similar peak activations that corresponded to previous AD reports.
Comparison with existing methods: The implemented architecture achieved a significant performance improvement over the classical support vector machine and the stacked autoencoder frameworks (p < 0.005), numerically better than state-of-the-art performance using sMRI data alone (> 7% than the second-best performing method) and within 1% of the state-of-the-art performance considering learning using multiple neuroimaging modalities as well.
Conclusions: The explored frameworks reflected the high potential of deep learning architectures in learning subtle predictive features and utility in critical applications such as predicting and understanding disease progression.
Keywords: Alzheimer's disease; Deep learning; MCI to AD progression; Residual neural networks.
Copyright © 2020 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest None.
Figures

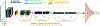

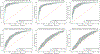



References
-
- Abrol A, Chaze C, Damaraju E, and Calhoun VD. 2016. “The Chronnectome: Evaluating Replicability of Dynamic Connectivity Patterns in 7500 Resting FMRI Datasets.” In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS. - PubMed
-
- “ADNI MRI Protocols.” 2017. http://adni.loni.usc.edu/methods/documents/mri-protocols (July 20, 2005).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

