Regulatory T cells promote remyelination in the murine experimental autoimmune encephalomyelitis model of multiple sclerosis following human neural stem cell transplant
- PMID: 32276110
- PMCID: PMC7253332
- DOI: 10.1016/j.nbd.2020.104868
Regulatory T cells promote remyelination in the murine experimental autoimmune encephalomyelitis model of multiple sclerosis following human neural stem cell transplant
Abstract
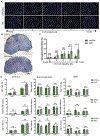
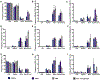
Multiple sclerosis (MS) is a chronic, inflammatory autoimmune disease that affects the central nervous system (CNS) for which there is no cure. In MS, encephalitogenic T cells infiltrate the CNS causing demyelination and neuroinflammation; however, little is known about the role of regulatory T cells (Tregs) in CNS tissue repair. Transplantation of neural stem and progenitor cells (NSCs and NPCs) is a promising therapeutic strategy to promote repair through cell replacement, although recent findings suggest transplanted NSCs also instruct endogenous repair mechanisms. We have recently described that dampened neuroinflammation and increased remyelination is correlated with emergence of Tregs following human NPC transplantation in a murine viral model of immune-mediated demyelination. In the current study we utilized the prototypic murine autoimmune model of demyelination experimental autoimmune encephalomyelitis (EAE) to test the efficacy of hNSC transplantation. Eight-week-old, male EAE mice receiving an intraspinal transplant of hNSCs during the chronic phase of disease displayed remyelination, dampened neuroinflammation, and an increase in CNS CD4+CD25+FoxP3+ regulatory T cells (Tregs). Importantly, ablation of Tregs abrogated histopathological improvement. Tregs are essential for maintenance of T cell homeostasis and prevention of autoimmunity, and an emerging role for Tregs in maintenance of tissue homeostasis through interactions with stem and progenitor cells has recently been suggested. The data presented here provide direct evidence for collaboration between CNS Tregs and hNSCs promoting remyelination.
Keywords: Multiple sclerosis; Neural stem cells; Neuroinflammation; Regulatory T cells; Remyelination.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors have no competing interests to disclose.
Figures





References
-
- Ben-Hur T, et al. , 2013. Cell-based reparative therapies for multiple sclerosis. Curr Neurol Neurosci Rep. 13, 397. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

