Sounds of seizures
- PMID: 32276233
- PMCID: PMC7269794
- DOI: 10.1016/j.seizure.2020.03.008
Sounds of seizures
Abstract
Purpose: A phase I feasibility study to determine the accuracy of identifying seizures based on audio recordings.
Methods: We systematically generated 166 audio clips of 30 s duration from 83 patients admitted to an epilepsy monitoring unit between 1/2015 and 12/2016, with one clip during a seizure period and one clip during a non-seizure control period for each patient. Five epileptologists performed a blinded review of the audio clips and rated whether a seizure occurred or not, and indicated the confidence level (low or high) of their rating. The accuracy of individual and consensus ratings were calculated.
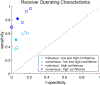
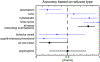
Results: The overall performance of the consensus rating between the five epileptologists showed a positive predictive value (PPV) of 0.91 and a negative predictive value (NPV) of 0.66. The performance improved when confidence was high (PPV of 0.96, NPV of 0.70). The agreement between the epileptologists was moderate with a kappa of 0.584. Hyperkinetic (PPV 0.92, NPV 0.86) and tonic-clonic (PPV and NPV 1.00) seizures were most accurately identified. Seizures with automatisms only and non-motor seizures could not be accurately identified. Specific seizure-related sounds associated with accurate identification included disordered breathing (PPV and NPV 1.00), rhythmic sounds (PPV 0.93, NPV 0.80), and ictal vocalizations (PPV 1.00, NPV 0.97).
Conclusion: This phase I feasibility study shows that epileptologists are able to accurately identify certain seizure types from audio recordings when the seizures produce sounds. This provides guidance for the development of audio-based seizure detection devices and demonstrate which seizure types could potentially be detected.
Keywords: Audio; Epilepsy; Seizure detection; Seizure monitor; Seizure safety.
Copyright © 2020 British Epilepsy Association. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest Author DF receives salary support for consulting and clinical trial related activities performed on behalf of The Epilepsy Study Consortium, a non-profit organization. DF receives no personal income for these activities. NYU receives a fixed amount from the Epilepsy Study Consortium towards DF’s salary. Within the past year, The Epilepsy Study Consortium received payments for research services performed by DF from: Adamas, Axcella, Biogen, Crossject, Engage Pharmaceuticals, Eisai, GW Pharmaceuticals, Pfizer, SK Life Science, Takeda, Xenon, and Zynerba. DF has also served as a paid consultant for Eisai. DF has received travel support from Medtronics, Eisai and the Epilepsy Foundation. DF receives research support from the CDC, NINDS, Epilepsy Foundation, Epitel, and Neuropace. DF serves on the scientific advisory board for Receptor Life Sciences. DF holds equity interests in Neuroview Technology and Receptor Life Sciences. Author RSF has done consulting for Medtronic and has stock options in Smart-Watch, Avails Medical, Cerebral Therapeutics, Zeto, Irody, Eysz. Author PD receives research support from the NIH and NeuroPace, Inc. PD has received honoraria for educational materials from NeuroPace, Inc. and travel reimbursement from Medtronic and NeuroPace, Inc. The remaining authors have no conflicts of interest.
Figures