Effect of Novel Pyrrolo[3,4- d]pyridazinone Derivatives on Lipopolysaccharide-Induced Neuroinflammation
- PMID: 32276316
- PMCID: PMC7177677
- DOI: 10.3390/ijms21072575
Effect of Novel Pyrrolo[3,4- d]pyridazinone Derivatives on Lipopolysaccharide-Induced Neuroinflammation
Abstract
Neuroinflammation is considered to be one of the potential causes for the development of neurodegenerative diseases, including Alzheimer's disease. In this study, we evaluated the effect of four newly synthesized pyrrolo[3,4-d]pyridazinone derivatives on the neuron-like PC12 cells under simulated inflammation conditions by preincubation with lipopolysaccharide (LPS). Our novel derivatives are selective cyclooxygenase-2 (COX-2) inhibitors and have similar effects to nonsteroidal anti-inflammatory drugs (NSAIDs). We assessed viability (LDH assay), metabolic activity (MTT assay), DNA damage (number of double-strand breaks measured by fast halo assay), and the neuronal features of cells (average neurite length and neurite outgrowth measured spectrofluorimetrically). DCF-DA and Griess assays were also performed, which allowed determining the impact of the tested compounds on the level of oxygen free radicals and nitrites. LPS administration significantly negatively affected the results in all tests performed, and treatment with the tested derivatives in most cases significantly reduced this negative impact. Multiple-criteria decision analysis indicated that overall, the best results were observed for compounds 2a and 2b at a concentration of 10 µM. The new derivatives showed intense activity against free oxygen radicals and nitrites. Reduced reactive oxygen species level also correlated with a decrease in the number of DNA damage. The compounds improved neuronal features, such as neurite length and outgrowth, and they also increased cell viability and mitochondrial activity. Our results suggest that derivatives 2a and 2b may also act additionally on mechanisms other than 3a and 3b.
Keywords: Alzheimer’s disease; LPS; NSAID; cyclooxygenase inhibitor; lipopolysaccharide; neuroinflammation; pyridazinone.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



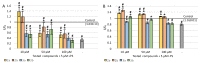




References
-
- Leszek J., Barreto G.E., Gąsiorowski K., Koutsouraki E., Ávila-Rodrigues M., Aliev G. Inflammatory mechanisms and oxidative stress as key factors responsible for progression of neurodegeneration: Role of brain innate immune system. CNS Neurol. Disord. - Drug Targets. 2016;15:329–336. doi: 10.2174/1871527315666160202125914. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

