A G-Protein Coupled Receptor and Macular Degeneration
- PMID: 32276449
- PMCID: PMC7226737
- DOI: 10.3390/cells9040910
A G-Protein Coupled Receptor and Macular Degeneration
Abstract
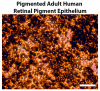
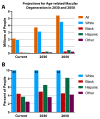
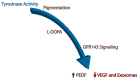
Age-related macular degeneration (AMD) is a leading cause of irreversible blindness in the world. The risk of AMD increases with age and is most common among the white population. Here, we discuss the convergence of factors related to race, pigmentation, and susceptibility to AMD, where the primary defect occurs in retinal support cells, the retinal pigment epithelium (RPE). We explore whether the observed racial bias in AMD incidence is related to innate differences in the basal level of pigmentation between races, and whether the pigmentation pathway activity in the RPE might protect from retinal degeneration. More specifically, we explore whether the downstream signaling activity of GPR143, a G-protein coupled receptor in the pigmentation pathway, might underly the racial bias of AMD and be a target to prevent the disease. Lastly, we summarize the past findings of a large retrospective study that investigated the relationship between the stimulation of GPR143 with L-DOPA, the pigmentation pathway, and AMD, to potentially help develop new ways to prevent or treat AMD. The reader of this review will come to understand the racial bias of AMD, which is related to the function of the RPE.
Keywords: AMD; G-protein; GPR143; L-DOPA; OA1; RPE; macular degeneration; melanin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Schmidt S.Y., Peisch R.D. Melanin concentration in normal human retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci. 1986;27:1063–1067. - PubMed
-
- Weiter J.J., Delori F.C., Wing G.L., Fitch K.A. Retinal pigment epithelial lipofuscin and melanin and choroidal melanin in human eyes. Invest. Ophthalmol. Vis. Sci. 1986;27:145–152. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

