Cumulative Effects of Social Stress on Reward-Guided Actions and Prefrontal Cortical Activity
- PMID: 32276717
- PMCID: PMC7434704
- DOI: 10.1016/j.biopsych.2020.02.008
Cumulative Effects of Social Stress on Reward-Guided Actions and Prefrontal Cortical Activity
Abstract
Background: When exposed to chronic social stress, animals display behavioral changes that are relevant to depressive-like phenotypes. However, the cascading relationship between incremental stress exposure and neural dysfunctions over time remains incompletely understood.
Methods: We characterized the longitudinal effect of social defeat on goal-directed actions and prefrontal cortical activity in mice using a novel head-fixed sucrose preference task and two-photon calcium imaging.
Results: Behaviorally, stress-induced loss of reward sensitivity intensifies over days. Motivational anhedonia, the failure to translate positive reinforcements into future actions, requires multiple sessions of stress exposure to become fully established. For neural activity, individual layer 2/3 pyramidal neurons in the cingulate and medial secondary motor subregions of the medial prefrontal cortex have heterogeneous responses to stress. Changes in ensemble activity differ significantly between susceptible and resilient mice after the first defeat session and continue to diverge following successive stress episodes before reaching persistent abnormal levels.
Conclusions: Collectively, these results demonstrate that the cumulative impact of an ethologically relevant stress can be observed at the level of cellular activity of individual prefrontal neurons. The distinct neural responses associated with resilience versus susceptibility suggests the hypothesis that the negative impact of social stress is neutralized in resilient animals, in part through an adaptive reorganization of prefrontal cortical activity.
Keywords: Chronic stress; Goal-directed behavior; Prefrontal cortex; Pyramidal neurons; Reward; Social defeat.
Copyright © 2020 Society of Biological Psychiatry. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
DISCLOSURES
The authors report no biomedical financial interests or potential conflicts of interest.
Figures





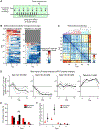
Comment in
-
Cumulative Stress Burden on Motivated Action Revealed.Biol Psychiatry. 2020 Oct 1;88(7):514-516. doi: 10.1016/j.biopsych.2020.07.010. Biol Psychiatry. 2020. PMID: 32912424 Free PMC article. No abstract available.
References
-
- Kendler KS, Karkowski LM, Prescott CA (1999): Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry. 156:837–841. - PubMed
-
- McEwen BS (2004): Protection and damage from acute and chronic stress: allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann N Y Acad Sci. 1032:1–7. - PubMed
-
- McEwen BS, Stellar E (1993): Stress and the individual. Mechanisms leading to disease. Arch Intern Med. 153:2093–2101. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

