Application of the 2017 KDIGO Guideline for the Evaluation and Care of Living Kidney Donors to Clinical Practice
- PMID: 32276946
- PMCID: PMC7274294
- DOI: 10.2215/CJN.12141019
Application of the 2017 KDIGO Guideline for the Evaluation and Care of Living Kidney Donors to Clinical Practice
Abstract
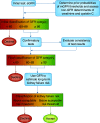
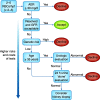
The Kidney Disease: Improving Global Outcomes (KDIGO) 2017 "Clinical Practice Guideline on the Evaluation and Care of Living Kidney Donors" was developed to assist medical professionals who evaluate living kidney donor candidates and provide care before, during, and after donation. This guideline Work Group concluded that a comprehensive approach to donor candidate risk assessment should replace eligibility decisions on the basis of assessments of single risk factors in isolation. To address all issues important to living donors in a pragmatic and comprehensive guideline, many of the guideline recommendations were on the basis of expert consensus opinion even when no direct evidence was available. To advance available evidence, original data analyses were also undertaken to produce a "proof-of-concept" risk projection model for kidney failure. This was done to illustrate how the community can advance a new quantitative framework of risk that considers each candidate's profile of demographic and health characteristics. A public review by stakeholders and subject matter experts as well as industry and professional organizations informed the final formulation of the guideline. This review highlights the guideline framework, key concepts, and recommendations, and uses five patient scenarios and 12 guideline statements to illustrate how the guideline can be applied to support living donor evaluation and care in clinical practice.
Keywords: Consensus; Data Analysis; Demography; Donor nephrectomy; Guideline; Kidney Diseases; Living Donors; Living donation; Patient-centered care; Renal Insufficiency; Risk Assessment; Risks; kidney; kidney transplantation; risk factors.
Copyright © 2020 by the American Society of Nephrology.
Figures


References
-
- Liyanage T, Ninomiya T, Jha V, Neal B, Patrice HM, Okpechi I, Zhao MH, Lv J, Garg AX, Knight J, Rodgers A, Gallagher M, Kotwal S, Cass A, Perkovic V: Worldwide access to treatment for end-stage kidney disease: A systematic review. Lancet 385: 1975–1982, 2015 - PubMed
-
- Tonelli M, Wiebe N, Knoll G, Bello A, Browne S, Jadhav D, Klarenbach S, Gill J: Systematic review: Kidney transplantation compared with dialysis in clinically relevant outcomes. Am J Transplant 11: 2093–2109, 2011 - PubMed
-
- Axelrod DA, Schnitzler MA, Xiao H, Irish W, Tuttle-Newhall E, Chang SH, Kasiske BL, Alhamad T, Lentine KL: An economic assessment of contemporary kidney transplant practice. Am J Transplant 18: 1168–1176, 2018 - PubMed
-
- Klarenbach S, Barnieh L, Gill J: Is living kidney donation the answer to the economic problem of end-stage renal disease? Semin Nephrol 29: 533–538, 2009 - PubMed
-
- Mahillo B, Carmona M, Álvarez M, White S, Noel L, Matesanz R: 2009 Global data in organ donation and transplantation: Activities, laws, and organization. Transplantation 92: 1069–1074, 2011 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

