Ecological Importance of Cross-Feeding of the Intermediate Metabolite 1,2-Propanediol between Bacterial Gut Symbionts
- PMID: 32276972
- PMCID: PMC7237793
- DOI: 10.1128/AEM.00190-20
Ecological Importance of Cross-Feeding of the Intermediate Metabolite 1,2-Propanediol between Bacterial Gut Symbionts
Abstract
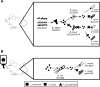
Cross-feeding based on the metabolite 1,2-propanediol has been proposed to have an important role in the establishment of trophic interactions among gut symbionts, but its ecological importance has not been empirically established. Here, we show that in vitro growth of Lactobacillus reuteri (syn. Limosilactobacillus reuteri) ATCC PTA 6475 is enhanced through 1,2-propanediol produced by Bifidobacterium breve UCC2003 and Escherichia coli MG1655 from the metabolization of fucose and rhamnose, respectively. Work with isogenic mutants showed that the trophic interaction is dependent on the pduCDE operon in L. reuteri, which encodes the ability to use 1,2-propanediol, and the l-fucose permease (fucP) gene in B. breve, which is required for 1,2-propanediol formation from fucose. Experiments in gnotobiotic mice revealed that, although the pduCDE operon bestows a fitness burden on L. reuteri ATCC PTA 6475 in the mouse digestive tract, the ecological performance of the strain was enhanced in the presence of B. breve UCC2003 and the mucus-degrading species Bifidobacterium bifidum The use of the respective pduCDE and fucP mutants of L. reuteri and B. breve in the mouse experiments indicated that the trophic interaction was specifically based on 1,2-propanediol. Overall, our work established the ecological importance of cross-feeding relationships based on 1,2-propanediol for the fitness of a bacterial symbiont in the vertebrate gut.IMPORTANCE Through experiments in gnotobiotic mice that employed isogenic mutants of bacterial strains that produce (Bifidobacterium breve) and utilize (Lactobacillus reuteri) 1,2-propanediol, this study provides mechanistic insight into the ecological ramifications of a trophic interaction between gut symbionts. The findings improve our understanding on how cross-feeding influences the competitive fitness of L. reuteri in the vertebrate gut and revealed a putative selective force that shaped the evolution of the species. The findings are relevant since they provide a basis to design rational microbial-based strategies to modulate gut ecosystems, which could employ mixtures of bacterial strains that establish trophic interactions or a personalized approach based on the ability of a resident microbiota to provide resources for the incoming microbe.
Keywords: 1,2-propanediol; Lactobacillus; bifidobacteria; competition; cross-feeding; fitness; gut microbiome; metabolism; microbial ecology; trophic interactions.
Copyright © 2020 American Society for Microbiology.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

