Intragenic repeat expansion in the cell wall protein gene HPF1 controls yeast chronological aging
- PMID: 32277013
- PMCID: PMC7263189
- DOI: 10.1101/gr.253351.119
Intragenic repeat expansion in the cell wall protein gene HPF1 controls yeast chronological aging
Abstract
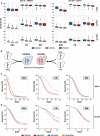
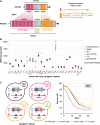
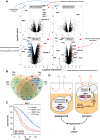
Aging varies among individuals due to both genetics and environment, but the underlying molecular mechanisms remain largely unknown. Using a highly recombined Saccharomyces cerevisiae population, we found 30 distinct quantitative trait loci (QTLs) that control chronological life span (CLS) in calorie-rich and calorie-restricted environments and under rapamycin exposure. Calorie restriction and rapamycin extended life span in virtually all genotypes but through different genetic variants. We tracked the two major QTLs to the cell wall glycoprotein genes FLO11 and HPF1 We found that massive expansion of intragenic tandem repeats within the N-terminal domain of HPF1 was sufficient to cause pronounced life span shortening. Life span impairment by HPF1 was buffered by rapamycin but not by calorie restriction. The HPF1 repeat expansion shifted yeast cells from a sedentary to a buoyant state, thereby increasing their exposure to surrounding oxygen. The higher oxygenation altered methionine, lipid, and purine metabolism, and inhibited quiescence, which explains the life span shortening. We conclude that fast-evolving intragenic repeat expansions can fundamentally change the relationship between cells and their environment with profound effects on cellular lifestyle and longevity.
© 2020 Barré et al.; Published by Cold Spring Harbor Laboratory Press.
Figures


Similar articles
-
Deleting the 14-3-3 protein Bmh1 extends life span in Saccharomyces cerevisiae by increasing stress response.Genetics. 2009 Dec;183(4):1373-84. doi: 10.1534/genetics.109.107797. Epub 2009 Oct 5. Genetics. 2009. PMID: 19805817 Free PMC article.
-
Extension of chronological life span in yeast by decreased TOR pathway signaling.Genes Dev. 2006 Jan 15;20(2):174-84. doi: 10.1101/gad.1381406. Genes Dev. 2006. PMID: 16418483 Free PMC article.
-
Autophagy is required for extension of yeast chronological life span by rapamycin.Autophagy. 2009 Aug;5(6):847-9. doi: 10.4161/auto.8824. Epub 2009 Aug 23. Autophagy. 2009. PMID: 19458476 Free PMC article.
-
The chronological life span of Saccharomyces cerevisiae.Aging Cell. 2003 Apr;2(2):73-81. doi: 10.1046/j.1474-9728.2003.00033.x. Aging Cell. 2003. PMID: 12882320 Review.
-
Chronological aging in Saccharomyces cerevisiae.Subcell Biochem. 2012;57:101-21. doi: 10.1007/978-94-007-2561-4_5. Subcell Biochem. 2012. PMID: 22094419 Free PMC article. Review.
Cited by
-
Evolution of natural lifespan variation and molecular strategies of extended lifespan in yeast.Elife. 2021 Nov 9;10:e64860. doi: 10.7554/eLife.64860. Elife. 2021. PMID: 34751131 Free PMC article.
-
HPF1 regulates tendon stem/progenitor cell senescence and tendon repair via PARP1-mediated poly-ADP ribosylation of HuR.Genes Genomics. 2024 Jan;46(1):27-36. doi: 10.1007/s13258-023-01447-w. Epub 2023 Sep 15. Genes Genomics. 2024. PMID: 37713069
-
Genomic Adaptations of Saccharomyces Genus to Wine Niche.Microorganisms. 2022 Sep 9;10(9):1811. doi: 10.3390/microorganisms10091811. Microorganisms. 2022. PMID: 36144411 Free PMC article. Review.
-
On the Ecological Significance of Phenotypic Heterogeneity in Microbial Populations Undergoing Starvation.Microbiol Spectr. 2022 Feb 23;10(1):e0045021. doi: 10.1128/spectrum.00450-21. Epub 2022 Jan 12. Microbiol Spectr. 2022. PMID: 35019773 Free PMC article.
-
Advancing genomic technologies and clinical awareness accelerates discovery of disease-associated tandem repeat sequences.Genome Res. 2022 Jan;32(1):1-27. doi: 10.1101/gr.269530.120. Epub 2021 Dec 29. Genome Res. 2022. PMID: 34965938 Free PMC article. Review.
References
-
- Arlia-Ciommo A, Leonov A, Beach A, Richard VR, Bourque SD, Burstein MT, Kyryakov P, Gomez-Perez A, Koupaki O, Feldman R, et al. 2018. Caloric restriction delays yeast chronological aging by remodeling carbohydrate and lipid metabolism, altering peroxisomal and mitochondrial functionalities, and postponing the onsets of apoptotic and liponecrotic modes of regulated cell death. Oncotarget 9: 16163–16184. 10.18632/oncotarget.24604 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
