A Method To Prevent SARS-CoV-2 IgM False Positives in Gold Immunochromatography and Enzyme-Linked Immunosorbent Assays
- PMID: 32277023
- PMCID: PMC7269408
- DOI: 10.1128/JCM.00375-20
A Method To Prevent SARS-CoV-2 IgM False Positives in Gold Immunochromatography and Enzyme-Linked Immunosorbent Assays
Abstract
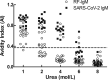
We set out to investigate the interference factors that led to false-positive novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) IgM detection results using gold immunochromatography assay (GICA) and enzyme-linked immunosorbent assay (ELISA) and the corresponding solutions. GICA and ELISA were used to detect SARS-CoV-2 IgM in 86 serum samples, including 5 influenza A virus (Flu A) IgM-positive sera, 5 influenza B virus (Flu B) IgM-positive sera, 5 Mycoplasma pneumoniae IgM-positive sera, 5 Legionella pneumophila IgM-positive sera, 6 sera of HIV infection patients, 36 rheumatoid factor IgM (RF-IgM)-positive sera, 5 sera from hypertensive patients, 5 sera from diabetes mellitus patients, and 14 sera from novel coronavirus infection disease 19 (COVID-19) patients. The interference factors causing false-positive reactivity with the two methods were analyzed, and the urea dissociation test was employed to dissociate the SARS-CoV-2 IgM-positive serum using the best dissociation concentration. The two methods detected positive SARS-CoV-2 IgM in 22 mid-to-high-level-RF-IgM-positive sera and 14 sera from COVID-19 patients; the other 50 sera were negative. At a urea dissociation concentration of 6 mol/liter, SARS-CoV-2 IgM results were positive in 1 mid-to-high-level-RF-IgM-positive serum and in 14 COVID-19 patient sera detected using GICA. At a urea dissociation concentration of 4 mol/liter and with affinity index (AI) levels lower than 0.371 set to negative, SARS-CoV-2 IgM results were positive in 3 mid-to-high-level-RF-IgM-positive sera and in 14 COVID-19 patient sera detected using ELISA. The presence of RF-IgM at mid-to-high levels could lead to false-positive reactivity of SARS-CoV-2 IgM detected using GICA and ELISA, and urea dissociation tests would be helpful in reducing SARS-CoV-2 IgM false-positive results.
Keywords: enzyme-linked immunosorbent assay; false-positive; gold immunochromatography assay; novel coronavirus; urea.
Copyright © 2020 Wang et al.
Figures

References
-
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W; China Novel Coronavirus Investigating and Research Team. 2020. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 382:727–733. doi:10.1056/NEJMoa2001017. - DOI - PMC - PubMed
-
- Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. 2020. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395:507–513. doi:10.1016/S0140-6736(20)30211-7. - DOI - PMC - PubMed
-
- Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DKW, Bleicker T, Brünink S, Schneider J, Schmidt ML, Mulders D, Haagmans BL, van der Veer B, van den Brink S, Wijsman L, Goderski G, Romette JL, Ellis J, Zambon M, Peiris M, Goossens H, Reusken C, Koopmans MPG, Drosten C. 22 January 2020, posting date Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2020.25.3..... - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

