The many lives of type IA topoisomerases
- PMID: 32277049
- PMCID: PMC7242696
- DOI: 10.1074/jbc.REV120.008286
The many lives of type IA topoisomerases
Abstract
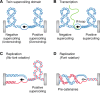
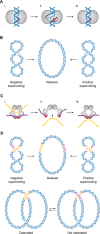
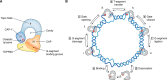
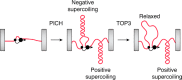
The double-helical structure of genomic DNA is both elegant and functional in that it serves both to protect vulnerable DNA bases and to facilitate DNA replication and compaction. However, these design advantages come at the cost of having to evolve and maintain a cellular machinery that can manipulate a long polymeric molecule that readily becomes topologically entangled whenever it has to be opened for translation, replication, or repair. If such a machinery fails to eliminate detrimental topological entanglements, utilization of the information stored in the DNA double helix is compromised. As a consequence, the use of B-form DNA as the carrier of genetic information must have co-evolved with a means to manipulate its complex topology. This duty is performed by DNA topoisomerases, which therefore are, unsurprisingly, ubiquitous in all kingdoms of life. In this review, we focus on how DNA topoisomerases catalyze their impressive range of DNA-conjuring tricks, with a particular emphasis on DNA topoisomerase III (TOP3). Once thought to be the most unremarkable of topoisomerases, the many lives of these type IA topoisomerases are now being progressively revealed. This research interest is driven by a realization that their substrate versatility and their ability to engage in intimate collaborations with translocases and other DNA-processing enzymes are far more extensive and impressive than was thought hitherto. This, coupled with the recent associations of TOP3s with developmental and neurological pathologies in humans, is clearly making us reconsider their undeserved reputation as being unexceptional enzymes.
Keywords: BLM; DNA replication; DNA supercoiling; DNA topology; DNA transcription; PICH; TOP3A; TOP3B; chromosome segregation; chromosomes; protein translocation; reverse gyrase; translocases.
© 2020 Bizard and Hickson.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures



References
-
- Naughton C., Avlonitis N., Corless S., Prendergast J. G., Mati I. K., Eijk P. P., Cockroft S. L., Bradley M., Ylstra B., and Gilbert N. (2013) Transcription forms and remodels supercoiling domains unfolding large-scale chromatin structures. Nat. Struct. Mol. Biol. 20, 387–395 10.1038/nsmb.2509 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

