Effects of neural estrogen receptor beta deletion on social and mood-related behaviors and underlying mechanisms in male mice
- PMID: 32277160
- PMCID: PMC7148327
- DOI: 10.1038/s41598-020-63427-4
Effects of neural estrogen receptor beta deletion on social and mood-related behaviors and underlying mechanisms in male mice
Abstract
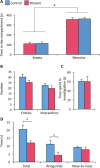
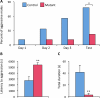
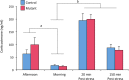
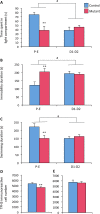
Estradiol derived from neural aromatization of testosterone plays a key role in the organization and activation of neural structures underlying male behaviors. This study evaluated the contribution of the estrogen receptor (ER) β in estradiol-induced modulation of social and mood-related behaviors by using mice lacking the ERβ gene in the nervous system. Mutant males exhibited reduced social interaction with same-sex congeners and impaired aggressive behavior. They also displayed increased locomotor activity, and reduced or unaffected anxiety-state level in three paradigms. However, when mice were exposed to unescapable stress in the forced swim and tail suspension tests, they spent more time immobile and a reduced time in swimming and climbing. These behavioral alterations were associated with unaffected circadian and restraint stress-induced corticosterone levels, and unchanged number of tryptophan hydroxylase 2-immunoreactive neurons in the dorsal raphe. By contrast, reduced mRNA levels of oxytocin and arginine-vasopressin were observed in the bed nucleus of stria terminalis, whereas no changes were detected in the hypothalamic paraventricular nucleus. The neural ERβ is thus involved to different extent levels in social and mood-related behaviors, with a particular action on oxytocin and arginine-vasopressin signaling pathways of the bed nucleus of stria terminalis, yet the involvement of other brain areas cannot be excluded.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

