Investigating lymphangiogenesis in a sacrificially bioprinted volumetric model of breast tumor tissue
- PMID: 32278014
- PMCID: PMC7541558
- DOI: 10.1016/j.ymeth.2020.04.003
Investigating lymphangiogenesis in a sacrificially bioprinted volumetric model of breast tumor tissue
Abstract
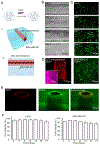
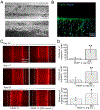
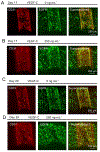
Lymphatic vessels, as a means to metastasize, are frequently recruited by tumor tissues during their progression. However, reliable in vitro models to dissect the intricate crosstalk between lymphatic vessels and tumors are still in urgent demand. Here, we describe a tissue-engineering method based on sacrificial bioprinting, to develop an enabling model of the human breast tumor with embedded multiscale lymphatic vessels, which is compatible with existing microscopy to examine the processes of lymphatic vessel sprouting and breast tumor cell migration in a physiologically relevant volumetric microenvironment. This platform will potentially help shed light on the complex biology of the tumor microenvironment, tumor lymphangiogenesis, lymphatic metastasis, as well as tumor anti-lymphangiogenic therapy in the future. We further anticipate wide adoption of the method to the production of various tissues and their models with incorporation of lymphatics vessels towards relevant applications.
Keywords: Angiogenesis; Bioprinting; Breast tumor; Lymphatic vessels; Sacrificial; Tumor microenvironment.
Copyright © 2020 Elsevier Inc. All rights reserved.
Figures

References
-
- Siegel RL, Miller KD, and Jemal A, CA Cancer J Clin, 70(1) (2020) 7–30. - PubMed
-
- Fidler IJ and Ellis LM, Cell, 79(2) (1994) 185–188. - PubMed
-
- Hanahan D and Weinberg RA, Cell, 144(5) (2011) 646–674. - PubMed
-
- Stacker SA, Williams SP, Karnezis T, Shayan R, Fox SB, and Achen MG, Nat Rev Cancer, 14(3) (2014) 159–172. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

